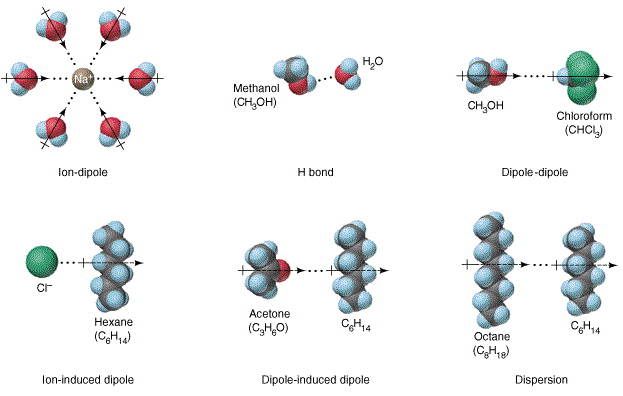
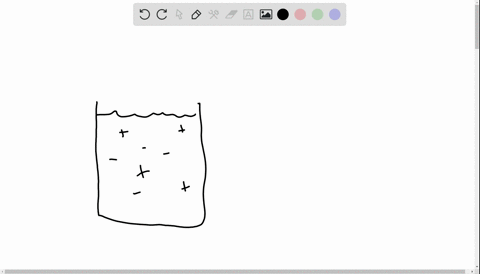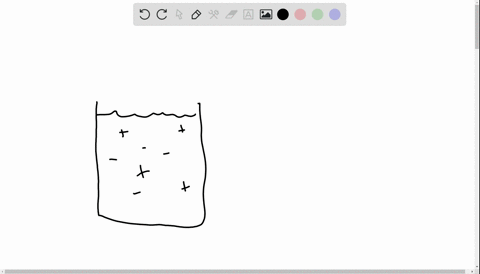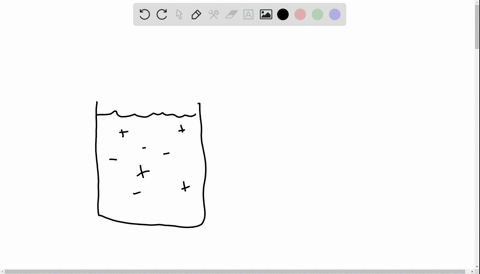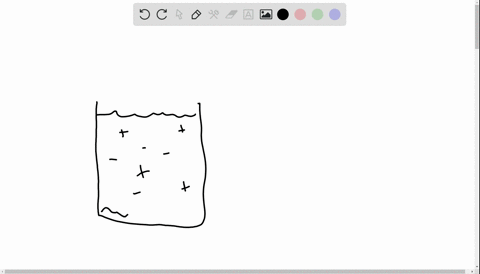
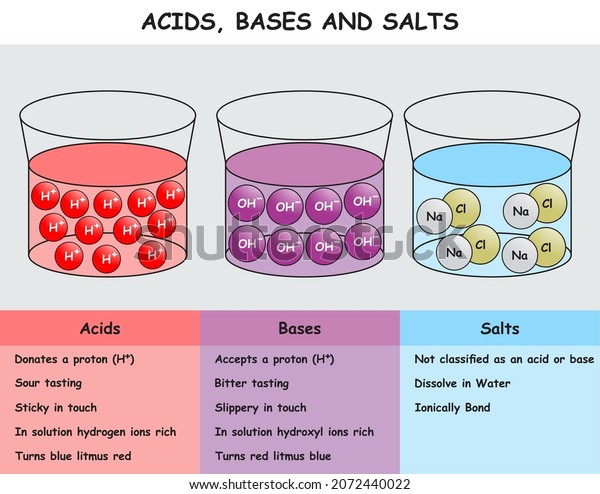
36 diagram of table salt dissolved in water
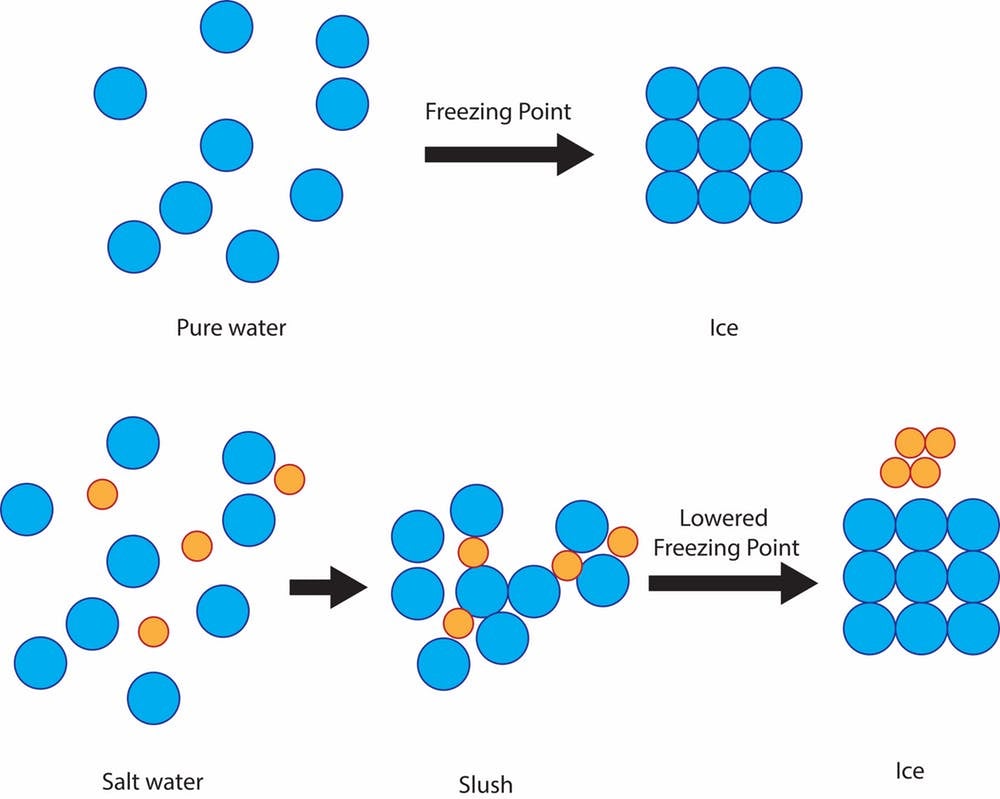
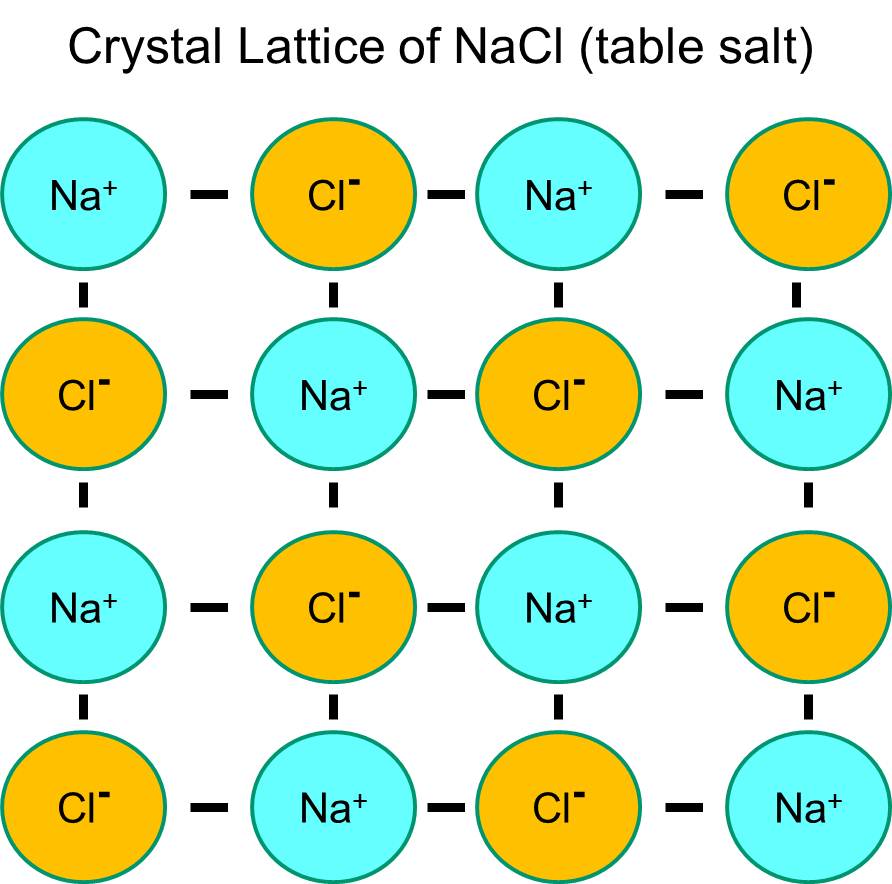
PDF Seawater Chemistry Lab - MiraCosta College (¼ teaspoon salt dissolved in 280mL water) has a salinity of about 6 parts per thousand (6.00I 00). You are now going to check the conductivity of water with half that salinity, or 3.00I 00. Pour half (140 mL) of the 6.00I 00 water sample from the beaker back into the 500mL graduated cylinder. Solubility - College of Saint Benedict and Saint John's ... You could easily dissolve about 360 g of table salt in a liter of water, but the solubility of calcium carbonate is only about 0.01 grams per liter. That's partly due to the fact that the ions in sodium chloride, Na + and Cl-, have lower charges than the ions in calcium carbonate, Ca 2+ and CO 3 2- .
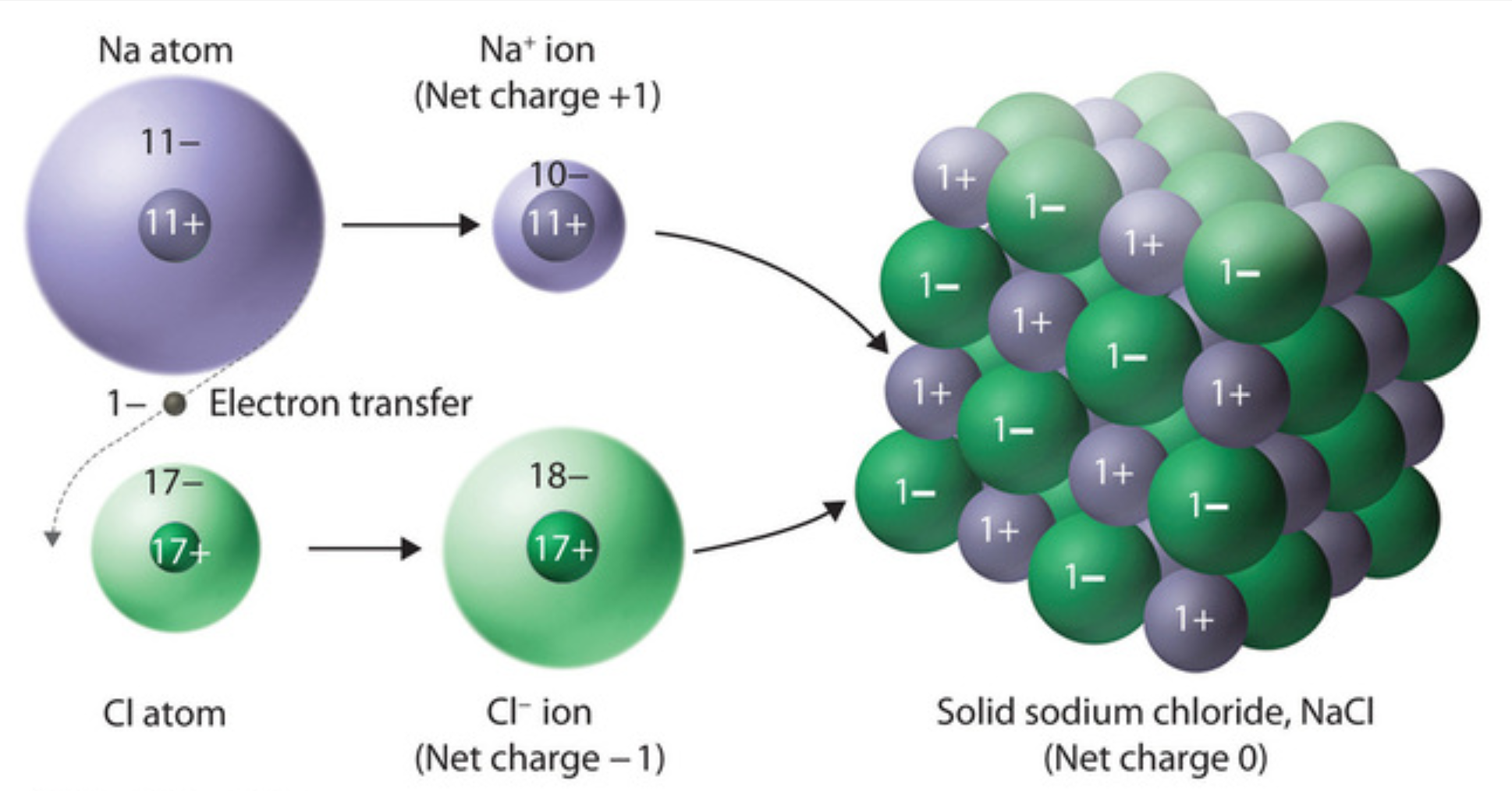
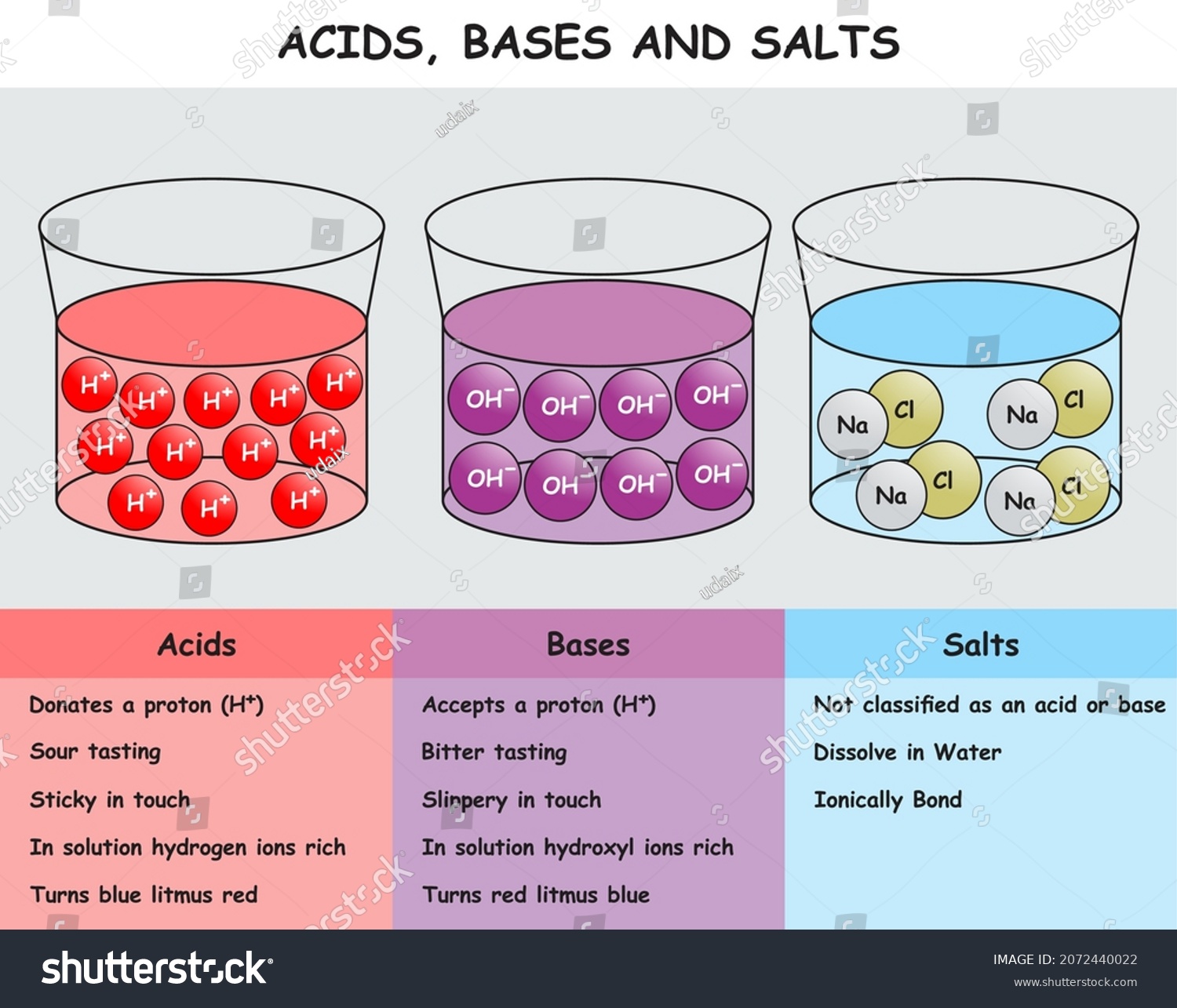
Sodium chloride - Wikipedia Sodium chloride / ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d /, commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible ...

Diagram of table salt dissolved in water
What Happens When Salt Is Added to Water? - Sciencing Table salt is made of the ionic compound sodium chloride, which consists of the chemical elements sodium and chlorine.You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; the more salt you add, the longer this takes, and it may require some shaking or stirring to bring about. Why Does Water Dissolve Salt? | Chapter 5 - Middle School ... Students will make a 2-D model of a salt crystal and use water molecule cut-outs to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Objective How Does Salt Dissolve in Water? - Reference.com Although common table salt easily dissolves in water, not all ionic salts do. If the strength of the attraction between the ions is much greater than the strength exerted by the slight charges of the water molecule, the ions remain bonded in water. A set of established rules, known as the solubility rules, provide the general guidelines and ...
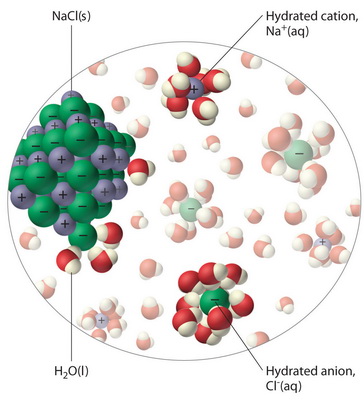
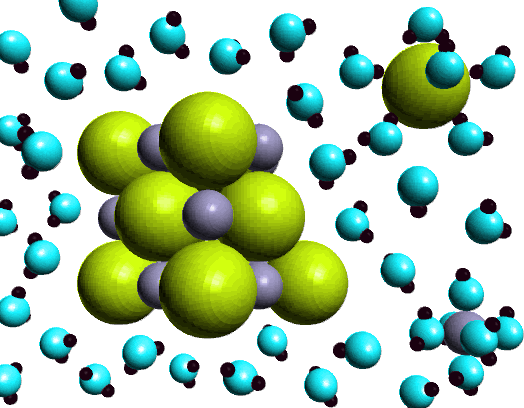
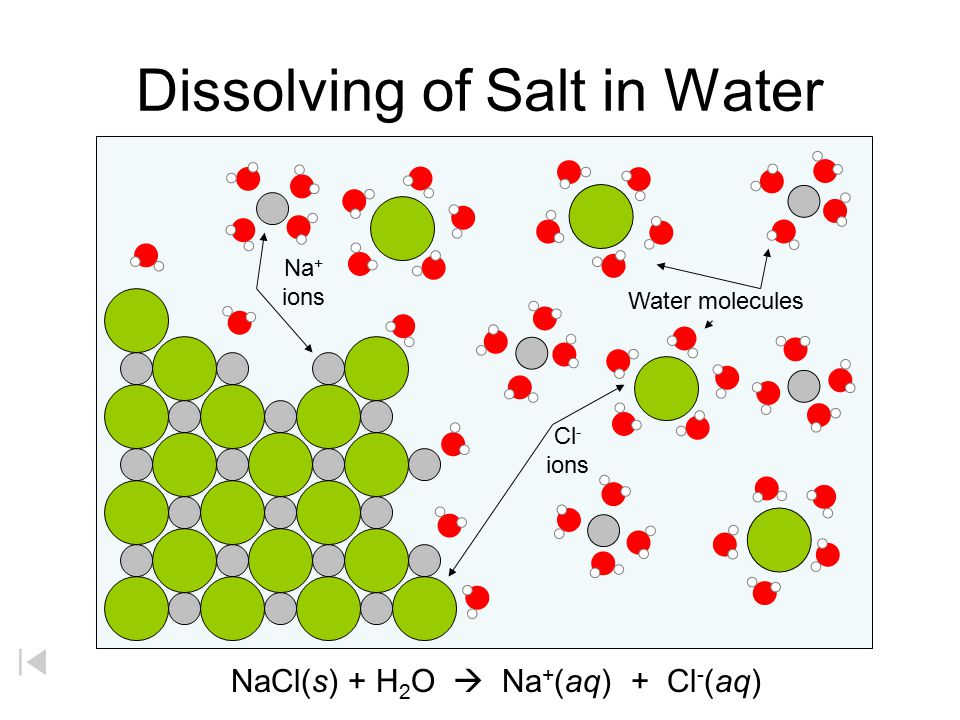
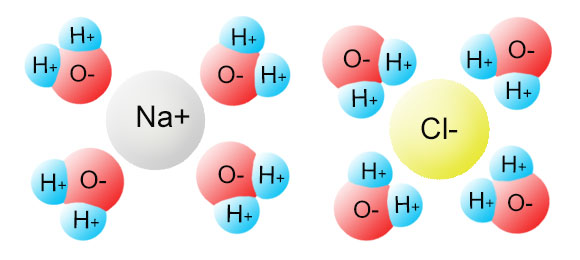
Diagram of table salt dissolved in water. Dissolved Oxygen and Water | U.S. Geological Survey Dissolved Oxygen and Water. Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in the water - the amount of oxygen available to living aquatic organisms. The amount of dissolved oxygen in a stream or lake can tell us a lot about its water quality. Sources/Usage: Public Domain. Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. UCSB Science Line Does salt dissolve quicker in room temperature than in cold water? Question Date: 2017-10-22: Answer 1: Dissolving is a process where solvent molecules surround other molecules, which causes the solid to become uniformly dispersed in a solution.In your example, salt ions in a solid are slowly surrounded by water molecules. PDF UNIVERSITY OF CAMBRIDGE INTERNATIONAL ... - XtremePapers 5 Two different solids, T and V, were analysed. T was a calcium salt. The tests on the solids and some of the observations are in the following table. Complete the observations in the table. tests observations white solid (a) tests on solid T Appearance of solid T. colour orange pH 5 [2] [2] (b) A little of solid T was dissolved in distilled water.
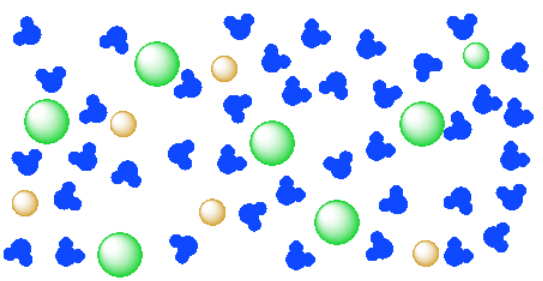
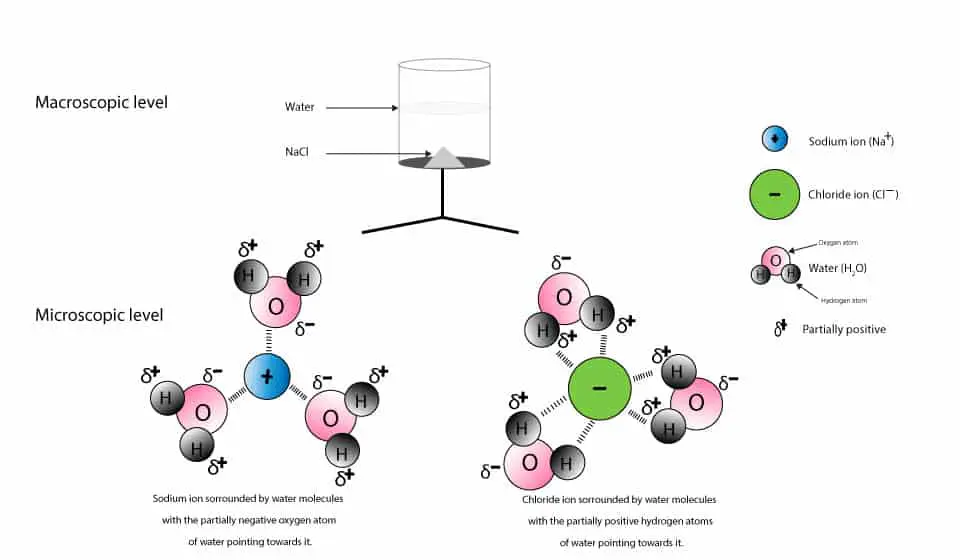
What type of mixture is salt dissolved in water? - All ... For example salt dissolving in water is usually considered to be a physical change, however the chemical species in salt solution (hydrated sodium and chlorine ions) are different from the species in solid salt. Is salt dissolved in water homogeneous? The salt dissolved in water is a homogeneous mixture, or a solution (Figure 3.5. 3). Answered: Exactly 1.00 grams of granular table… | bartleby Exactly 1.00 grams of granular table salt was dissolved in deionized water up to 500 mL. An aliquot of 20 mL was titrated with AgNO3 (T = 8244 mg/mL) and consumed 46.60 mL to reach end point. If the impurities are just water, calculate the percent Moisture. Ans in 1 sig figure. What happens if NaCl (table salt) is mixed with water? - Quora Answer (1 of 10): Table salt is an ionic compound . Its molecule is made of positive sodium ions (Na+) and negative (Cl-) ions. The polarity of water molecules enables water to dissolve many ionically bonded substances. The positive part of water molecules attracts the negative chloride ions and... what happens when nacl is dissolved in water - Lisbdnet.com What Happens When Nacl Is Dissolved In Water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a ...
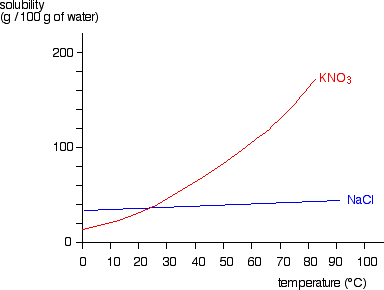
Untitled [faculty.uml.edu] Overview . In this experiment, you attempted to determine the solubility of a salt (KNO 3) at various temperatures, and plotted a solubility curve for the salt. The solubility of the salt refers to the mass of the salt which will dissolve per 100 mL of solvent (in this case, water) at a particular temperature. To do this, you took a fixed amount of salt, and determined at what temperature the ... How does water dissolve salts - Florida State College at ... These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes. Please Watch animation 10.3 on ionic solutions. Draw a diagram of table salt (NaCl) dissolved in water ... Find step-by-step Biology solutions and your answer to the following textbook question: Draw a diagram of table salt (NaCl) dissolved in water..1 answer · Top answer: $\textbf{\color{#c34632}Explanation:-}$ $\Rightarrow$ Water molecules ionize NaCl molecules and convert them into positive sodium ions and negative ... How to Dissolve Salt in Water: 9 Steps (with Pictures ... Measure out a fixed amount of water (e.g., 1 liter). Add salt one gram at a time, stirring until it completely dissolves. Repeat until no more will dissolve. With table salt and pure water, you should get around 350-360 grams of salt per liter of water to dissolve.
What Is an Aqueous Solution? - Easy ... - Easy Hard Science Take a look at the below science diagram. This is how we represent salt water. It's an aqueous solution of plain old table salt, chemical formula NaCl for sodium chloride, dissolved in water.First, note there is lots of water, H2O, shown with oxygen atoms in red and hydrogen atoms in gray.
SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. Video Transcript. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared ...
Dissolving Salt in Water - msnucleus.org Cool water molecules are tighter together and will not allow much salt to dissolve. PROCEDURE: Students will make salt from water. In this lab ordinary table salt will be used, but in reality the "salt" in salt water consists of various other compounds as will be discussed in the post lab. Water can just take so much salt. Have the students ...
Dissolution of NaCl in Water - interactive simulations ... If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ...
44 diagram of salt dissolved in water - Wiring Diagram Source Diagram of salt dissolved in water Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Dissolving Salt in Water: Chemical or Physical Change? When you dissolve table salt (sodium chloride, also known as NaCl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a chemical reaction, with new substances produced as a result of the change.A physical change, on the other hand, results in a change of the material's appearance, but no new chemical products result.
How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
What happens when sodium chloride NaCl is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Solved A) Draw two molecules of MgCl2 dissolved in water ... Question: A) Draw two molecules of MgCl2 dissolved in water (show at least 6 water molecules and their interactions with each other and with the salt ions). (HINT: MgCl2 is a salt and made with ionic bonds). In your diagram, label at least one hydrogen and one polar-covalent bond. B) Silicon has said to be an alternative to carbon and possibly ...
PDF ELECTROLYSIS OF SALT WATER - NASA Aquarius Mission ELECTROLYSIS OF SALT WATER Unit: Salinity Patterns & the Water Cycle l Grade Level: High school l Time Required: Two 45 min. periods l Content Standard: NSES Physical Science, properties and changes of properties in matter; atoms have measurable properties such as electrical charge. l Ocean Literacy Principle 1e: Most of of Earth's water (97%) is in the ocean.
How Does Salt Dissolve in Water? - Reference.com Although common table salt easily dissolves in water, not all ionic salts do. If the strength of the attraction between the ions is much greater than the strength exerted by the slight charges of the water molecule, the ions remain bonded in water. A set of established rules, known as the solubility rules, provide the general guidelines and ...
Why Does Water Dissolve Salt? | Chapter 5 - Middle School ... Students will make a 2-D model of a salt crystal and use water molecule cut-outs to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Objective
What Happens When Salt Is Added to Water? - Sciencing Table salt is made of the ionic compound sodium chloride, which consists of the chemical elements sodium and chlorine.You probably learned from unintentional play at the kitchen table as a child that if you sprinkle salt into a glass of pure water, the salt disappears after a time; the more salt you add, the longer this takes, and it may require some shaking or stirring to bring about.















0 Response to "36 diagram of table salt dissolved in water"
Post a Comment