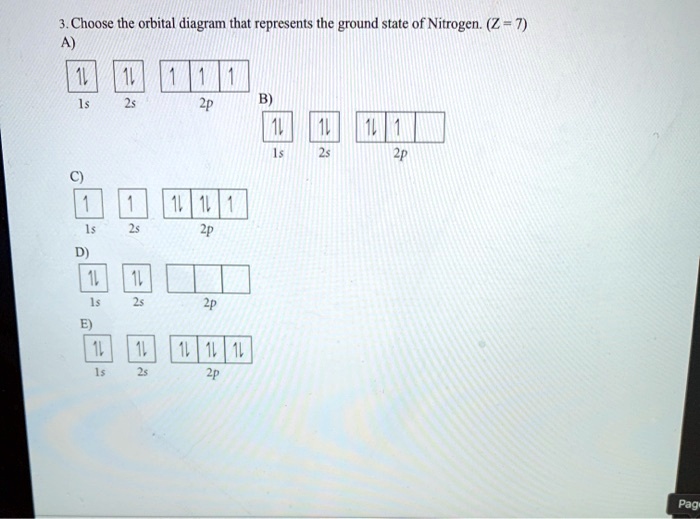
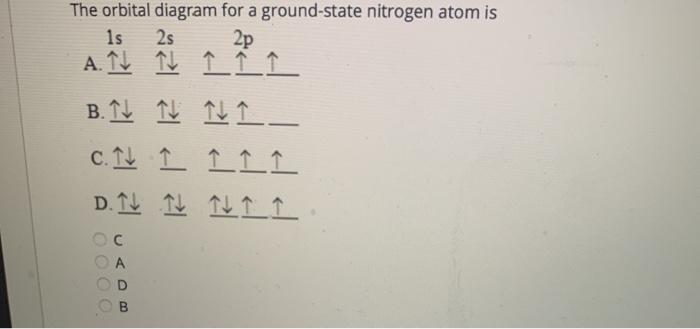
39 the orbital diagram for a ground state nitrogen atom is
topblogtenz.com › sulfur-orbital-diagram-electronSulfur Orbital diagram, Electron configuration, and Valence ... There is a simple difference between Ground state and Excited-state configuration. The ground state configuration of an atom is the same as its regular electron configuration in which electrons remain in the lowest possible energy. So, the ground-state electron configuration for the Sulfur atom is 1s 2 2s 2 2p 6 3s 2 3p 4. topblogtenz.com › chlorine-orbital-diagramChlorine Orbital diagram, Electron configuration, and Valence ... There is a simple difference between Ground state and Excited-state configuration. The ground state configuration of an atom is the same as its regular electron configuration in which electrons remain in the lowest possible energy. So, the ground-state electron configuration for the Chlorine atom is 1s 2 2s 2 2p 6 3s 2 3p 5.
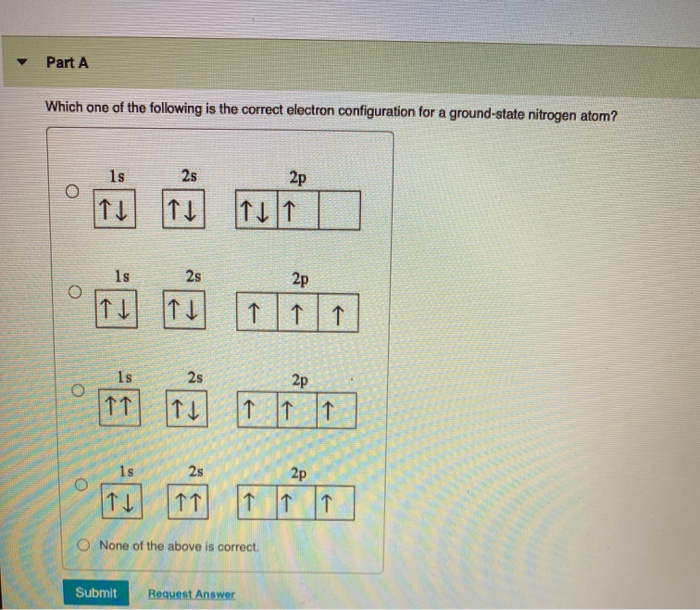
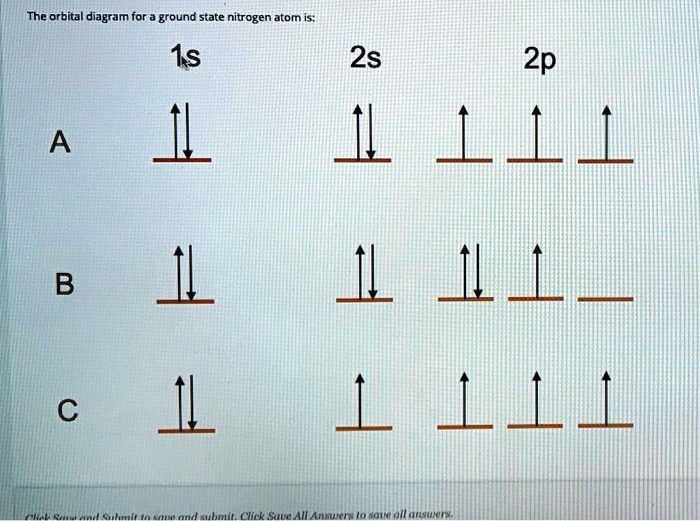
HW #3 Flashcards - Quizlet The electron configuration of a ground-state Co atom is ... 1s2,2s2,2p6,3s2,3d9 [Ar]4s2,3d7 [Ne]3s2,3d7 [Ar]4s1,3d5 ... The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A ↿⇂ ↿⇂ ↿ . ↿ . ↿ B ↿⇂ ↿ . ↿⇂ ↿ . C ↿⇂ ↿⇂ ↿ . ↿ . ↿
The orbital diagram for a ground state nitrogen atom is
Ch 7/8 quiz Flashcards | Quizlet The orbital diagram for a ground-state nitrogen atom is. 1s (up down) 2s (up down) 2p (up, up, up) Electrons in an orbital with l = 3 are in a/an. ... For all atoms of the same element, the 2s orbital is larger than the 1s orbital. true. An FM radio station broadcasts at a frequency of 101.7 MHz. Calculate the wavelength EOF SOLVED:The orbital diagram for a ground-state nitrogen ... The orbital diagram for a ground-state nitrogen atom is 1s A_ 4 _l B 1 _1_ cf 1 LLL D 1 1 1LL Get the answer to your homework problem. Try Numerade Free for 7 Days
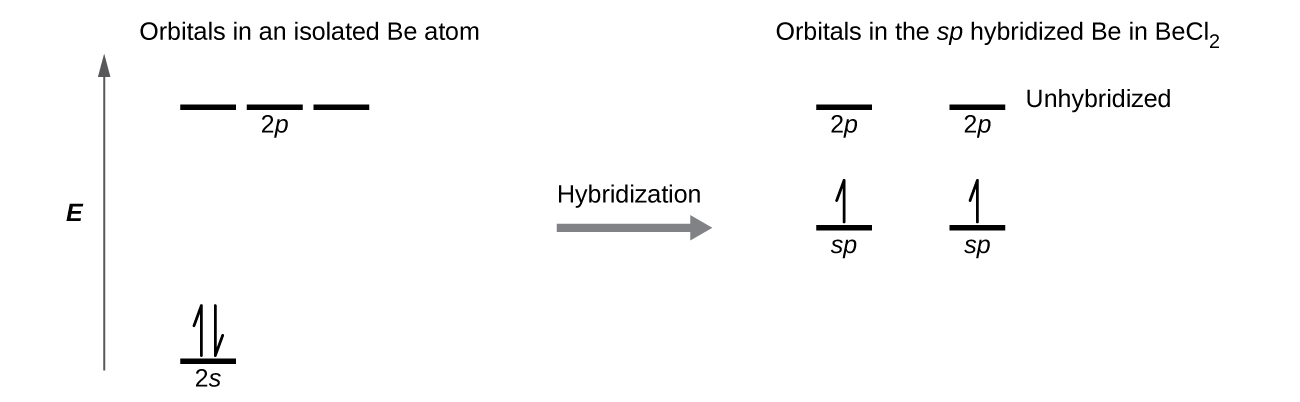
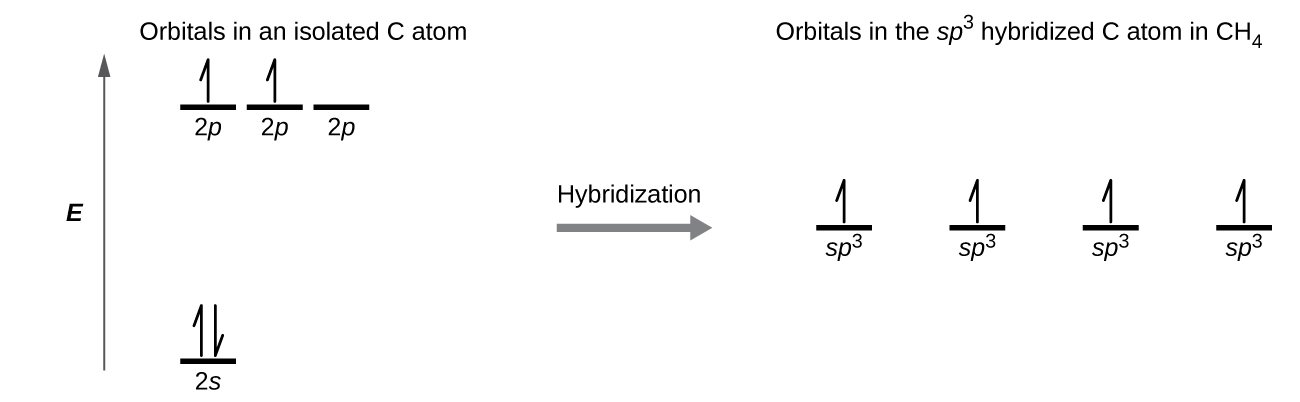
The orbital diagram for a ground state nitrogen atom is. en.wikipedia.org › wiki › Orbital_hybridisationOrbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. Chapter 2 Flashcards | Quizlet 5. 1 The orbital diagram for a ground state nitrogen (7) atom is: A. 2 The orbital diagram for a ground state oxygen (8) atom is: D. 3 The orbital diagram for a ground state carbon (6) atom is: D. 4 Which ground state atom has an electron configuration described by the following orbital diagram? Phosphorus. Exam 3 Study Guide Flashcards | Quizlet The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑ The orbital diagram for a ground state carbon atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ How many unpaired electrons does a ground-state atom of sulfur have? 2. Solved The orbital diagram for a ground state carbon atom ... The orbital diagram for a ground state carbon atom is 1s 2s A) A B) B C) D D) C Calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n-4 to the n-1 principal energy level. Recall that for hydrogen En -一2.18 × 10-18 J (1/n2) A) 0.612 nm B) 97.2 nm C) 82.6 nm D) 365 nm E) 6.8 × 10-18 nm.
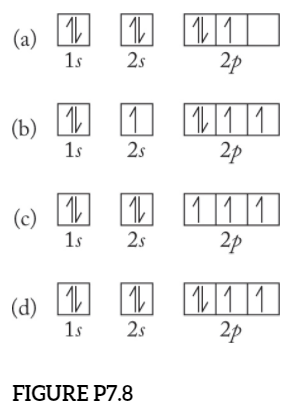
Solved The orbital diagram for a ground-state nitrogen ... The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소 ; Question: The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소 What is the orbital diagram for a ground-state nitrogen ... A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy. Chemistry Chapter 7 Flashcards & Practice Test - Quizlet The orbital diagram for a ground-state nitrogen atom is A) A B) B C) C D) D ... The orbital diagram for a ground state carbon atom is A) A B) B C) C D) D. D. section 7.9 A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga) in its ground state is A) A B) B C) C D) D E) E. Solved The orbital diagram for a ground-state nitrogen ... We review their content and use your feedback to keep the quality high. Option (A) is the correct orbital diagram for a ground -state nitrogen atom . Electronic configuration of nitrogen in ground state is, 1s22s22p3 or 1s22s22px12 …. View the full answer.
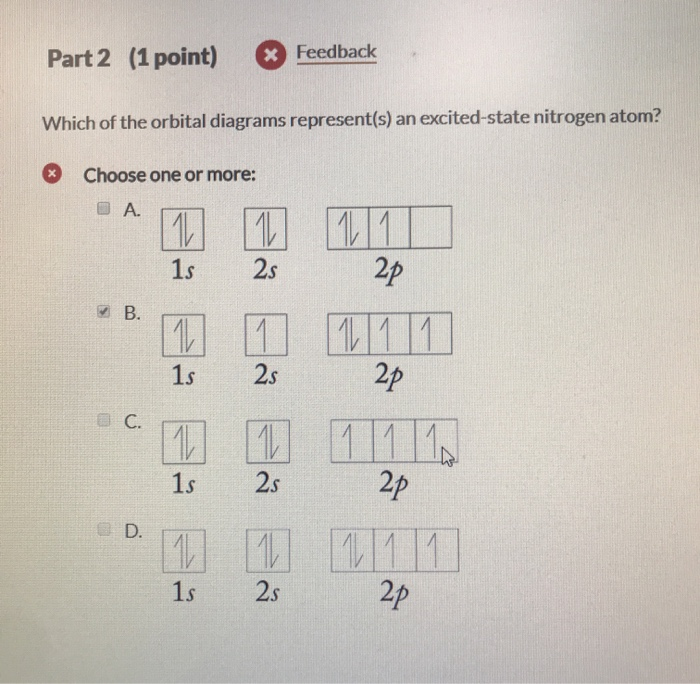
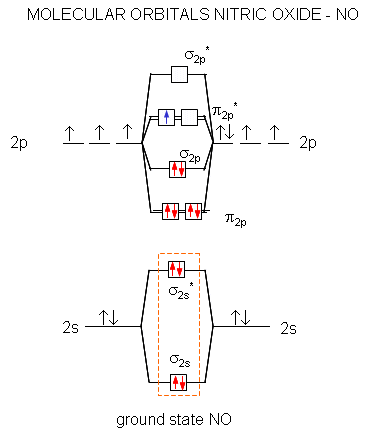
en.wikipedia.org › wiki › Molecular_orbital_diagramMolecular orbital diagram - Wikipedia A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. MO diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization. They also give insight to the bond order of the molecule, how many bonds are shared between the two atoms. Chapter 7 Chem Flashcards | Quizlet ground state- lowest energy state of the atom Aufbau- electrons fill orbitals starting with the lowest energy orbitals Paull exclusion- maximum of two electrons can occupy each orbital and they must have opposite spins Hund's- electrons are distributed into orbitals of identical energy in such a way as to give the maximum number of unpaired ... › chemistry › shapes-of-orbitalsShapes of Orbitals | What is Orbital? Types of Orbitals An ion or atom with one or more electrons occupies the higher energy orbitals and it is said to be in an excited state, whereas an ion or atom in which one or more electrons occupy low energy orbitals is said to be in its ground state. The Shape of Orbitals. A large number of orbitals occupy an atom. SOLVED:The orbital diagram for ground state nitrogen atom ... The orbital diagram for ground state nitrogen atom is: 1s 2s 2p 1 L1l B 1 , 1L I_ c L L11
valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
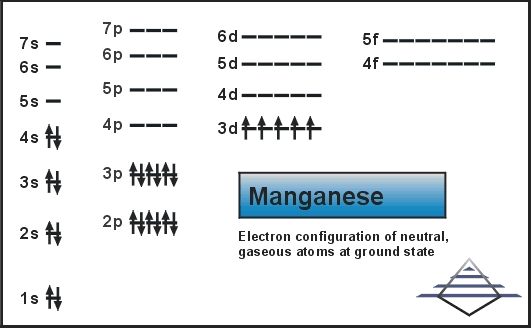
the ground state valence shell electrons configuration of ... the ground state valence shell electrons configuration of nitrogen atom can be represnted as :
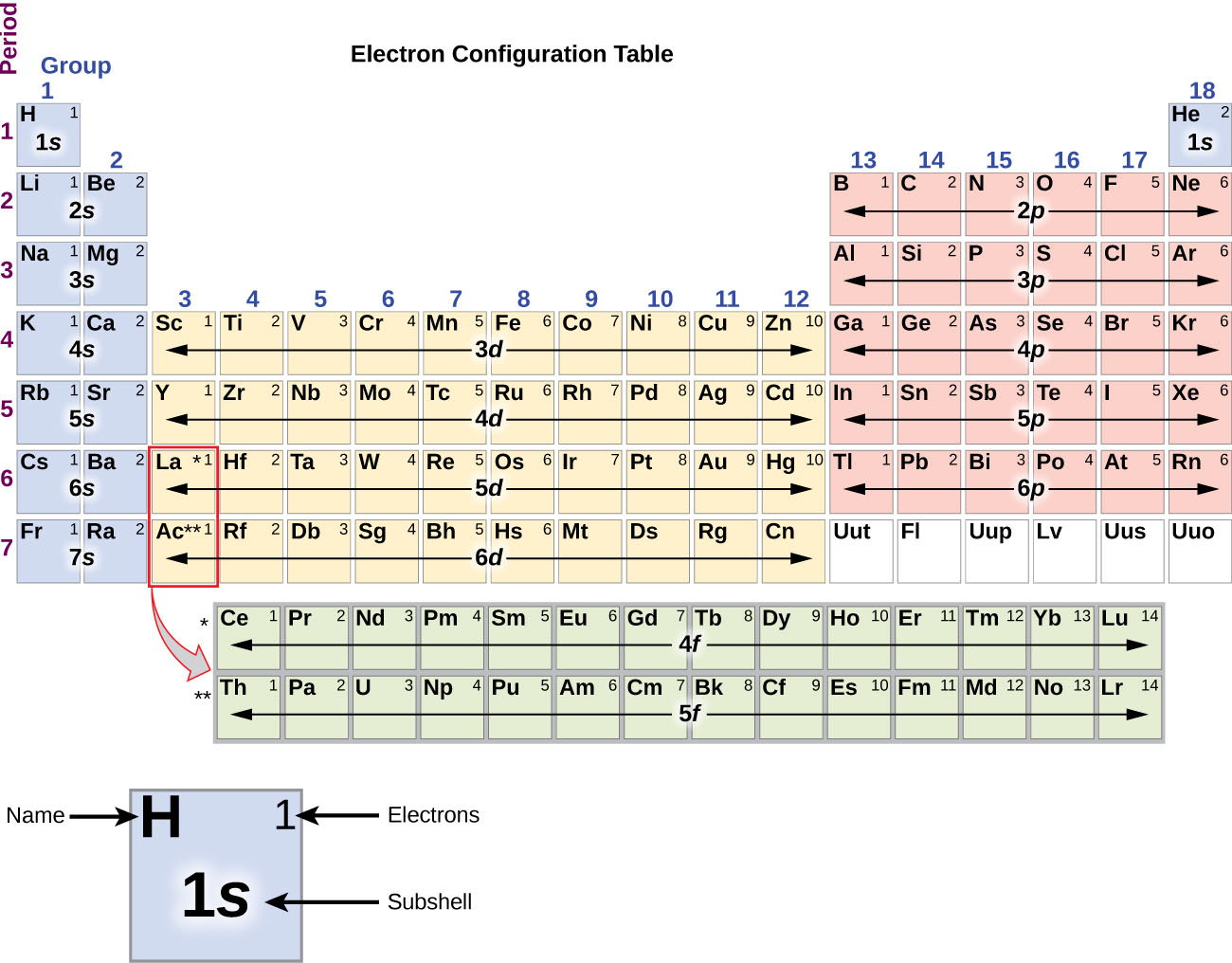
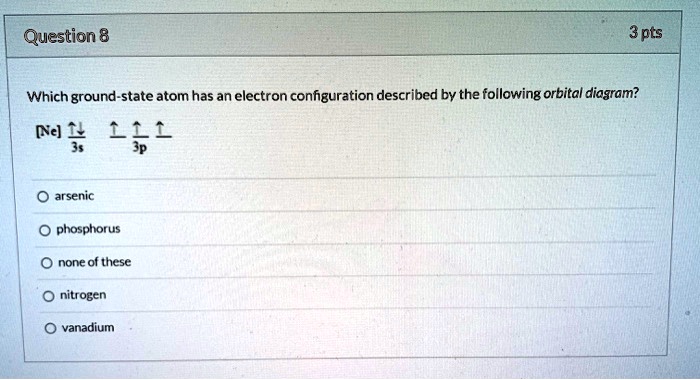
22The orbital diagram for a ground state nitrogen atom is ... 22the orbital diagram for a ground state nitrogen. This preview shows page 3 - 4 out of 4 pages. 23. Which ground-state atom has an electron configuration described by the following orbital diagram? [Ar] 4s 3d 4p (A) phosphorus (B) germanium (C) selenium (D) tellurium (E) none of these.
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
› cbse › mcq-questions-for-classMCQ Questions for Class 11 Chemistry Chapter 2 ... - Learn Cram Jun 17, 2021 · If the nitrogen atom had electronic configuration 1s², it would have energy lower than that of the normal ground state configuration 1s² 2s² 2p³, because the electrons would be closer to the nucleus. Yet, 1s² is not observed because it isolates. (a) Heisenberg’s Uncertainty Principle (b) Hund’s rule (c) Pauli Exclusion Principle
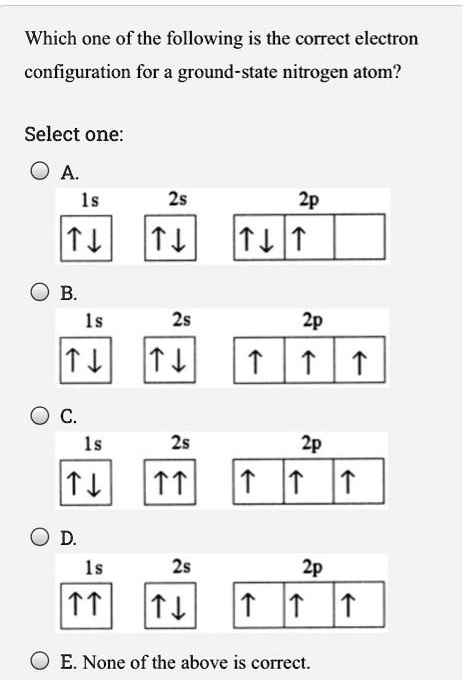
CHEM Final Flashcards - Quizlet Which electron orbital diagram is written correctly for an atom without any violations? ^ ^ ^^^ Which is the correct electron configuration for a nitrogen atom? 1s22s22p3. Which is the correct electron configuration for an oxide ion? ... What is the principal quantum number for the outermost electrons in a Te atom in the ground state? 5.
SOLVED:The orbital diagram for a ground-state nitrogen ... The orbital diagram for a ground-state nitrogen atom is 1s A_ 4 _l B 1 _1_ cf 1 LLL D 1 1 1LL Get the answer to your homework problem. Try Numerade Free for 7 Days
EOF
Ch 7/8 quiz Flashcards | Quizlet The orbital diagram for a ground-state nitrogen atom is. 1s (up down) 2s (up down) 2p (up, up, up) Electrons in an orbital with l = 3 are in a/an. ... For all atoms of the same element, the 2s orbital is larger than the 1s orbital. true. An FM radio station broadcasts at a frequency of 101.7 MHz. Calculate the wavelength






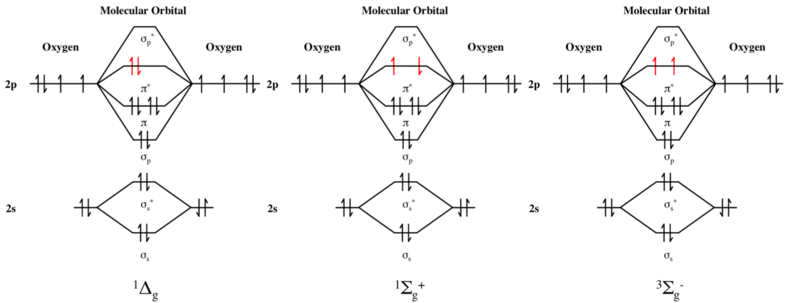














0 Response to "39 the orbital diagram for a ground state nitrogen atom is"
Post a Comment