36 in an electron dot diagram, the symbol for an element is used to represent
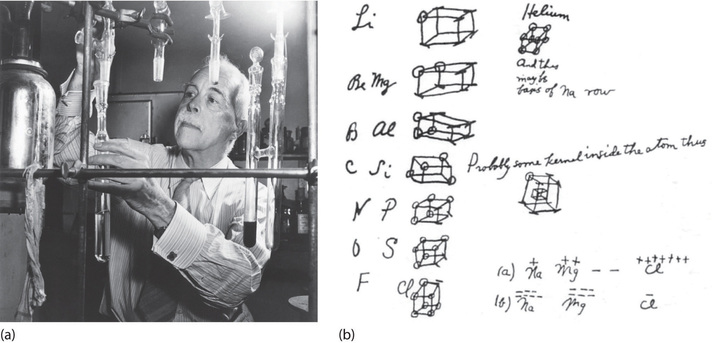
in an electron dot diagram, the symbol for an element is used to represent what? ... you see a structural formula in which the symbols for elements are connected by a long dash. you can assume that the chemical bonds in the compound are what? ... the symbol for an element is used to represent what? The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
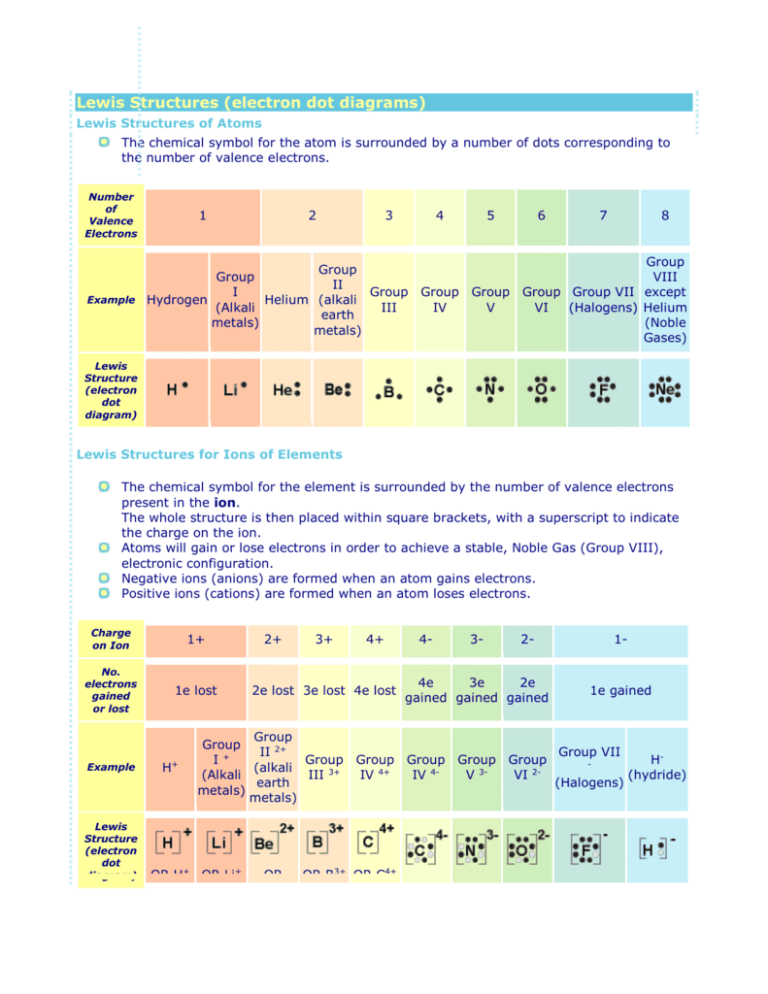
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, …
In an electron dot diagram, the symbol for an element is used to represent
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Transcribed image text: In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The ... In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The nucleus and all valence electrons d. The nucleus and all non-valence electrons. Choose the statement that correctly identifies the most stable of the following elements: lithium, carbon, fluorine, and neon a. Lithium ...
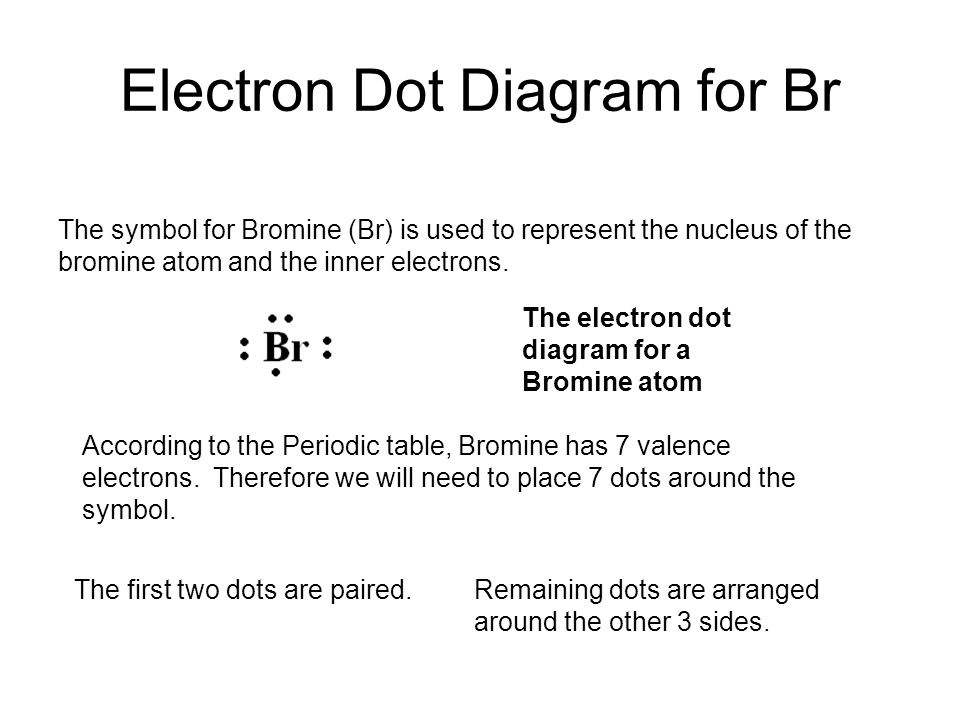
In an electron dot diagram, the symbol for an element is used to represent. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. In an electron dot diagram, the symbol for an element is used to represent the nucleus and all nonvalence electrons. Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure 6-1. In an electron dot diagram, the symbol for an element is used to represent the nucleus. For example : As we know that arsenic has '5' valence electrons. So, the symbol (As) is used to represent the nucleus and valance electrons around the is represented by the 'dot'. The Lewis-dot structure of As (arsenic) is shown below. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Electron dot diagrams would be the same for each element in the representative element groups. Click to see full answer.
Mar 30, 2018 — In an electron dot diagram, the symbol for an element is used to represent the nucleus. For example : As we know that arsenic has '5' valence ...2 answers · 10 votes: A the nucleus bruh it can't be anything else The electron-dot symbol for Mg shows two valence electrons as single dots on the sides of the symbol Mg. What does a dot in a dot diagram stand for? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The symbol in an Electron Dot Diagram is used to represent the nucleus and all the non-valence electrons, The Electron Dot Diagram is also known as the Lewis Dot Symbol. The Lewis Dot Symbol or Electron Dot Diagram, is a symbol of an element with one or more dots representing the valence electrons of an element. It was suggested by Gilbert N ... In this diagram, the delta symbol (δ) is used with a (+) or (-) symbol to represent partial positive and partial negative charge distribution in polar covalent bonds. Note that the electrons shared in polar covalent bonds will be attracted to and spend more time around the …
In an electron-dot symbol of an element, the dots are used to represent _____. a. all of the electrons in the atom b. the valence electrons A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Procedure. 1. Write the symbol for the element. For electron dot diagrams, this symbol represents the nucleus and all of the electrons of the atom except the outermost electrons. The symbol for chlorine is Cl. In an electron dot diagram, this symbol represents the nucleus and the ten electrons in the first two energy levels. 2. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Click to see full answer.
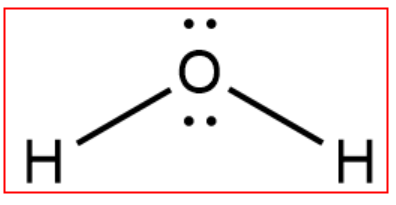
A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation.
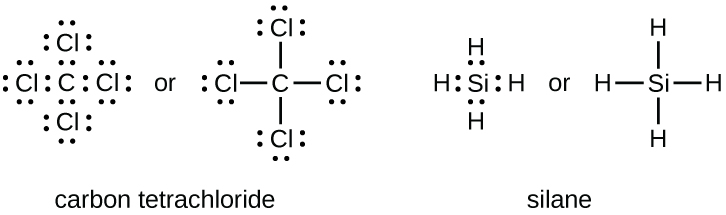
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
In an electron dot diagram the symbol for an element is used to represent what? In a Lews dot structure, the elemental symbol is used to represent the molecule's nucleus. The electrons are then ...
17.11.2021 · Molecular orbital diagram practice worksheet
Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = 4 shell, there are six ...
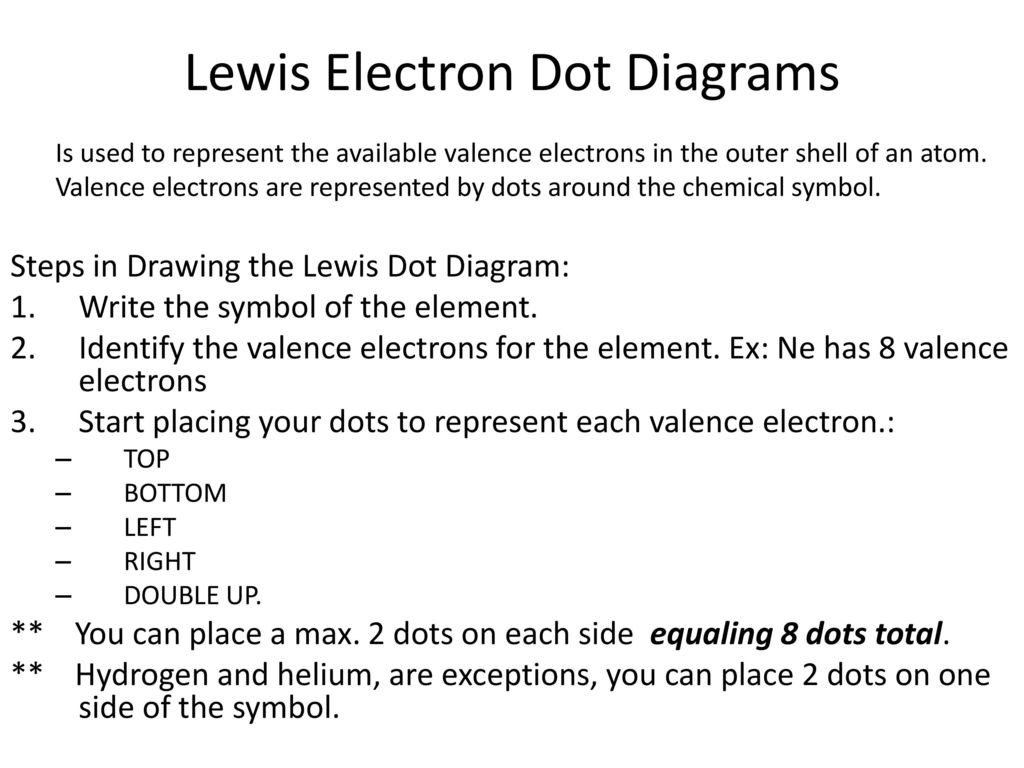
1. Write the symbol for the element. The symbol represents the nucleus and the inner or core electrons for the element. In the image below, a generic symbol, X, is used. There are four sides surrounding the symbol. 2. Determine the number of valence electrons for the element. Use a dot to represent an electron. 3.
Electron dot diagrams are used to represent the Lewis model of the arrangement of electrons in atoms. Which of the following statements regarding electron dot diagrams is incorrect? Select one: a. The element symbol represents the nucleus and filled energy levels of the atom. O b. Dots represent the valence electrons of the atom. c.

Use Electron Dots And Or Pairs Of Dots As Appropriate To Show The Lewis Symbol For The Neutral Atom Arsenic Study Com
In the Lewis Dot Structure, the element symbol represents the nucleus and inner electrons, and the surrounding dots represent the valence electrons.1 answer · Top answer: The problem asked what the dots in an electron-dot structure represent.Let's define an electron-dot structure:Lewis Dot Structures or Electron Dot Structures ...

A Lewis Dot Diagram Is An Easy Way To Represent An Atom S Valence Electrons Using Dots Around The Element S Symbol Lewis Dot Diagram Bohr Model Ppt Download
Again, it does not matter on which sides of the symbol the electron dots are positioned. For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. . As such, the electron dot diagram for ...
01.09.2021 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Sep 23, 2021 — Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis dot symbols can be used to predict the number ...
• Electron configuration describes the distribution of electrons among the various orbitals in the atom • Electron configuration is represented in two ways – The s, p, d, f notation – An orbital diagram C 1s22s22p2 Mn 1s22s22p63s23p64s23d5 3d 4s When an atom gains an electron to become a negatively-charged ion this is indicated by a minus sign after the element symbol; for example, F-.
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.

Difference Between Lewis Dot Symbol And Lewis Structure Compare The Difference Between Similar Terms
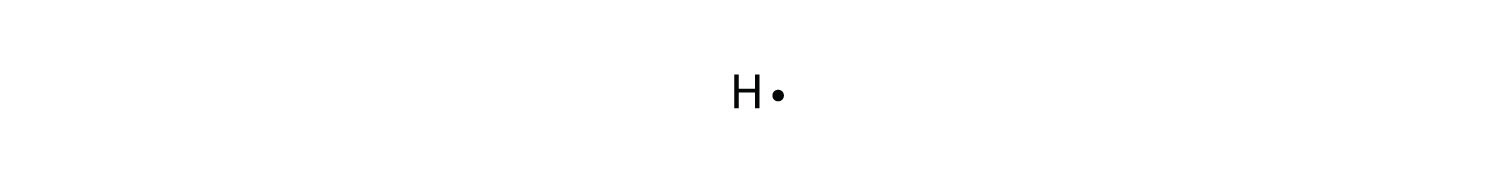
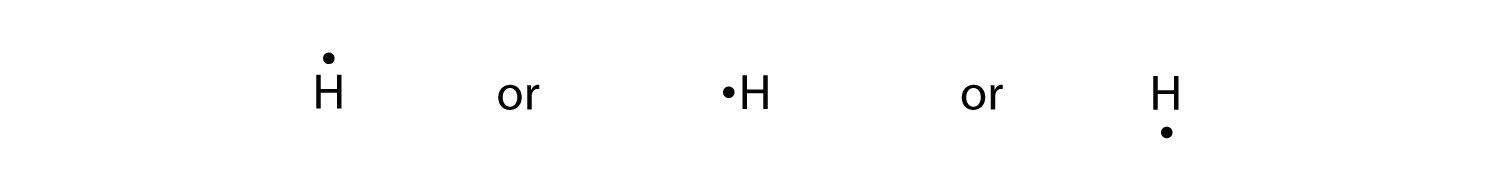
The graphical notation used for valence electrons is called an Electron-Dot Symbol. To draw an electron dot symbol, start with the abbreviation for the element of interest as the center, signifying the nucleus of the atom. From there, identify the number of valence electrons the atom has, and then add a single dot for each electron around the ...
2 дн. назад · In an electron dot diagram, the symbol for an element is used to represent the nucleus and all non-valence electrons. Log in for more information. Added 2 minutes 21 seconds ago|11/24/2021 3:32:18 PM
Correct answers: 3 question: In an electron dot diagram, the symbol for an element is used to represent
Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule
In an electron dot diagram, the symbol for an element is used to represent. In an electron dot diagram, the symbol for an element is used to represent. Post navigation. Previous Post. Previous Between 1993 and 1995 sodium phosphate was added to Seathwaite Tarn in the English Lake District to increase the Tarn's. Next Post.
In an electron dot diagram, the symbol for an element is used to represent A. the nucleus. B. the nucleus and all electrons. C. the nucleus and valence electrons. D. the nucleus and all non-valence electrons.
What does an electron dot structure show? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
To write an element's Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element's chemical symbol. Up to four dots ...
Electron dot diagrams, which are also called Lewis dot diagrams, are very useful tools in Chemistry. They will give you the ability to determine the type(s) of covalent bonds that an element may make in certain situations. They can also be used to predict the type of ion that an atom might make when it forms an ion.
In an electron dot diagram, the symbol for an element is used to represent the nucleus and all nonvalance electrons. Log in for more information. Added 9/6/2015 6:48:53 PM. This answer has been confirmed as correct and helpful.
A lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. A lewis dot diagram for an atom consists of the atomic symbol for the atom surrounded by dots that represent that atoms valence electrons.
In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The nucleus and all valence electrons d. The nucleus and all non-valence electrons. Choose the statement that correctly identifies the most stable of the following elements: lithium, carbon, fluorine, and neon a. Lithium ...
Transcribed image text: In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The ...
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.





/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

/lewisnitrite-56a128825f9b58b7d0bc90cf.jpg)











0 Response to "36 in an electron dot diagram, the symbol for an element is used to represent"
Post a Comment