36 orbital diagram for magnesium ion
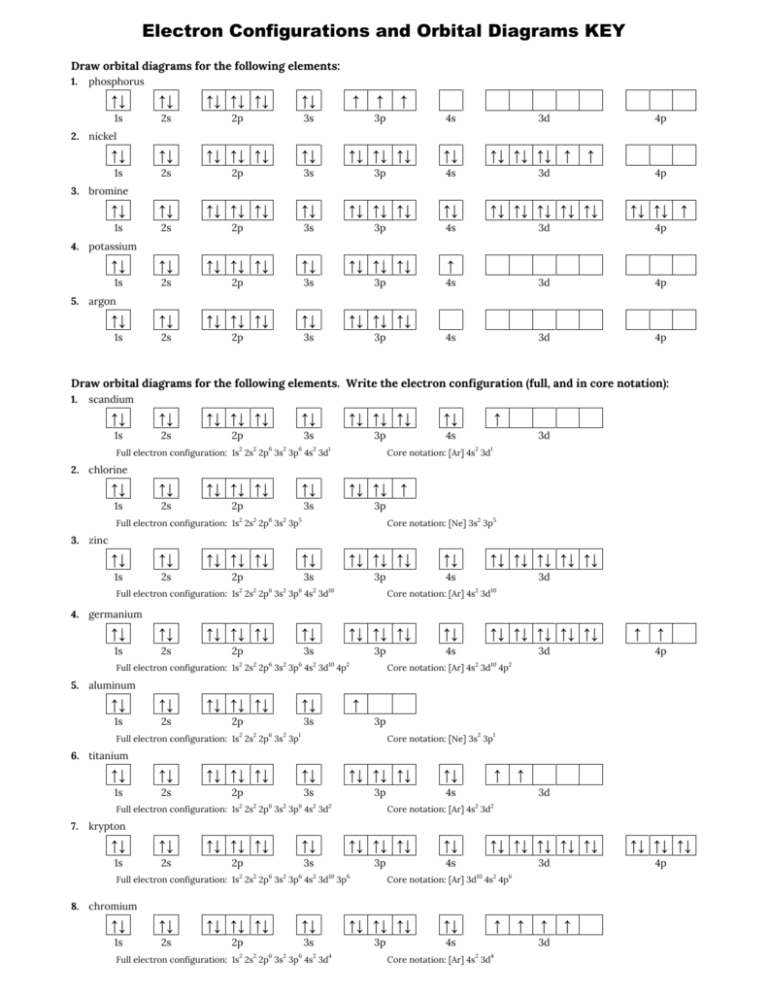
The electron configuration of a neutral magnesium atom is: 1s2 2s2 2p6 3s2 or in shorthand [Ne] 3s2. Magnesium has 2 valence (outer-shell) electrons and will lose both to fulfill the octet rule ... The lewis dot structure for magnesium is an mg with 2 dots which stand for its two valence electrons. What is an bond electron transfer how electron dot diagram for s awesome sulfur atom flow block orbital diagram for magnesium awesome lewis electron dot diagrams. Magnesium reacts with sulfur to produce sulfide a in.
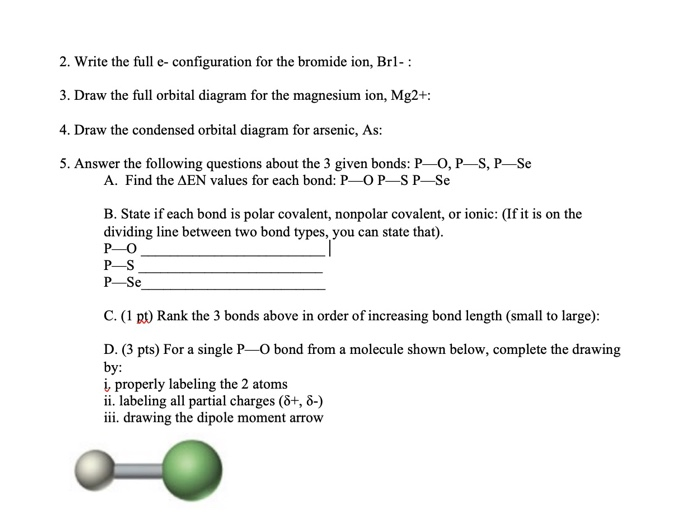
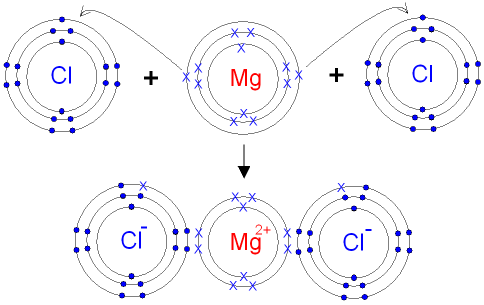
That is, magnesium is a cation element. Magnesium donates the electron of the last shell to form bonds and turns into magnesium ions. Mg - 2e - → Mg +2. The electron configuration of magnesium ions is 1s 2 2s 2 2p 6. The electron configuration of magnesium-ion shows that magnesium ions have only two shells and that shell has eight electrons.

Orbital diagram for magnesium ion
Answer to Draw an orbital diagram for each element: (a) magnesium; (b) aluminum; (c) bromine.. How can we draw the orbital diagram of a magnesium atom and a magnesium ion. What is the orbital diagram for magnesium. 100 10 ratings or. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements. Use the buttons at the top of the tool to add sublevels. The p orbital can hold up to six electrons. This video shows how to draw the orbital diagram of selenium (Se). It also shows how to write the electron configuration of selenium (Se) and the shorthand ...
Orbital diagram for magnesium ion. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. One may also ask, what does mg2+ represent? Mg2+ is a Mg atom that now has the same number of electrons as a noble gas. It achiveved this by ... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11. A step-by-step description of how to write the electron configuration for Magnesium (Mg). In order to write the Mg electron configuration we first need to kn... In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We’ll also look at why Magnesium forms a 2+ ion and how the electron con...
Draw the abbreviated orbital diagram for the iron ion in [Fe ... A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively. A graph of the successive ionization energies of magnesium is shown below. For MgCl2 we have an ionic compound and we need to take that into account when we draw the Lewis Structure. We’ll first draw the metal and put it in brackets... Mo3+ Orbital Diagram. Compact version of orbital energy diagram with each orbital represented . Mo2+, Mo3+, Mo4+ and Mo5+ are all known in aqueous solution. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. Ti ti2 ti4 construct the orbital diagram of each atom or ion. In this case titanium ti is located in period 4 group 4 of the periodic table and has an atomic number of 22. Write the corresponding electron configuration for. Construct the orbital diagram of the f ion. Draw the orbital diagram for ion ca 2. Get more help from chegg.
We're being asked to determine the correct electron configuration of a Magnesium (Mg) ion.. Before we can do that, we have to first write the electron configuration of a neutral ground state Mg. You can determine the ground-state electron configuration of Mg by locating the position in the periodic table.. Ground-state means that the element is in its lowest energy form (not in excited state). However, for atoms with three or fewer electrons in the p orbitals (Li through N) we observe a different pattern, in which the σp orbital is higher in energy than the πp set. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram ... Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. (a). Write down the electronic configuration of (i) magnesium atom, and (ii) magnesium ion, (At No. of Mg=12) ,brgt (b). Write down the electronic configurat...
How can we draw the orbital diagram of a magnesium atom and a magnesium ion. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom mg. Use the buttons at the top of the tool to add sublevels. The nex six electrons will go in the 2p orbital. Construct an orbital diagram to show the electron configuration ...
Solved Construct An Orbital Diagram To Show The Electron Configuration For A Neutral Magnesium Atom Mg Mg Drag The Appropriate Labels To Their Res Course Hero
Atomic Orbital Diagram of Calcium (2+) Ion. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. How to Write the Electron Configuration for Calcium (Ca)
One magnesium atom loses two electrons, to become a +2 ion (cation).Two chlorine atoms gain one electron each to become two -1 ions (anions).These are held t...
Orbital energy diagrams are provided to guide you learn about the atomic orbital. A chemical equation shows the chemical formulas of substances that are reacting and the substances that are produced. Depict the electron configuration for magnesium ing an orbital box diagram and noble gas notation.
We know that many atoms gain or lose electrons to achieve the same number of electrons as the nearest noble gas. We can now make a more general statement about the formation of monatomic ions · Certain monatomic ions form when atoms gain or lose electrons to achieve a stable electron configuration ...
Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ...
Click here👆to get an answer to your question ✍️ Give orbital diagram of the following:magnesium chloride,
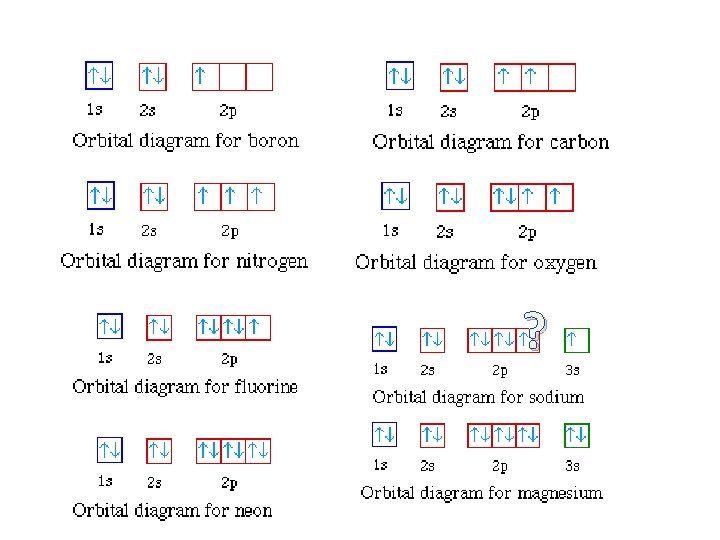
The electron configuration of the carbon atom represented by the orbital diagram is (see picture) This electron configuration can be written as 1s22s22p2 where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. ... Build the orbital diagram for the ion most likely formed by ...
13 Jan 2015 — Magnesium has an atomic number of 12, which means its neutral atom has 12 electrons. The "Mg"^(2+) ion is formed when the neutral magnesium atom ...1 answer · Yes, the "Mg"^(2+) ion and the neutral neon atom are isoelectronic, which implies that they have the same number of electrons and, of course, the same ...
Chem4Kids.com! Magnesium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
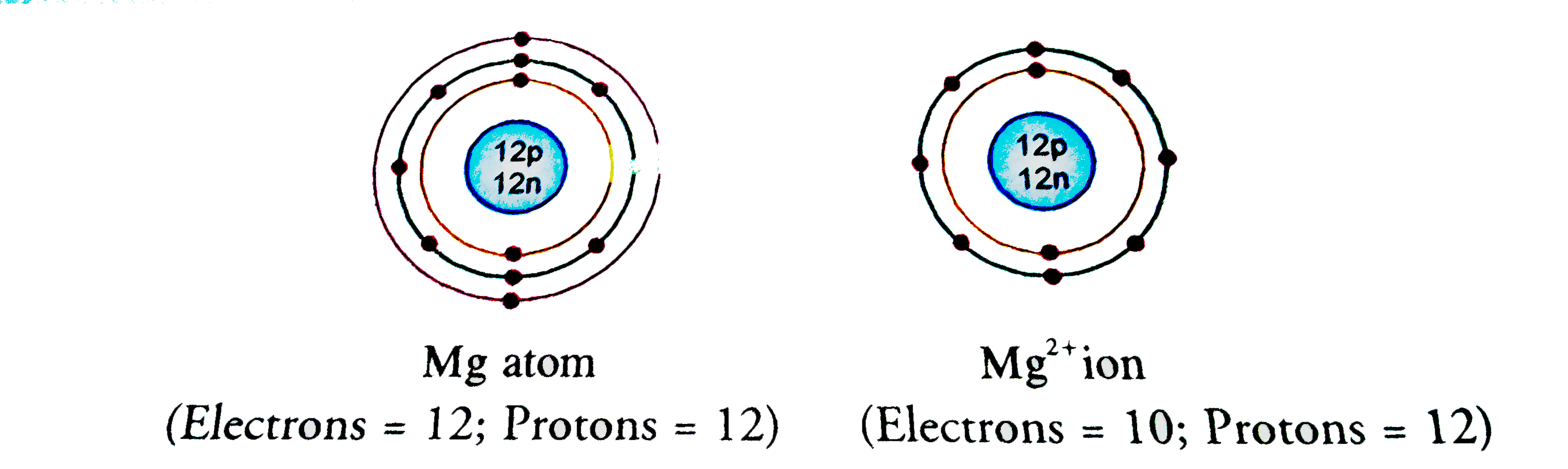
Show The Electron Distribution In Magnesium Atom And Magnesium Ion Diagrammatically And Also Give Their Atomic Numbers
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
July 17, 2020 - We can use energy-level diagrams such as the one in Figure \(\PageIndex{2}\) to describe the bonding in other pairs of atoms and ions where n = 1, such as the H2+ ion, the He2+ ion, and the He2 molecule. Again, we fill the lowest-energy molecular orbitals first while being sure not to violate ...

Electron Configurations Distributedexplains How Electrons Are Distributed Among An Atom S Orbitals Address Each Part Identifies Part Of An Electron S Address Ppt Download
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
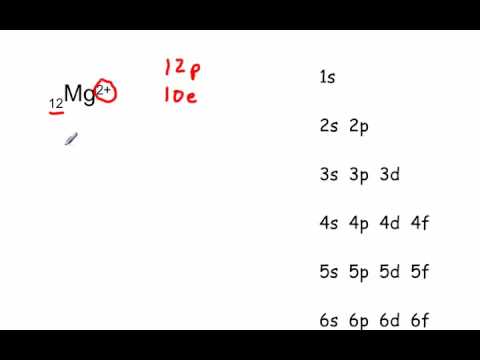
Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹.
Transcribed image text: Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
The electronic configuration for magnesium is 1s^2 2s^2 2p^6 3s^2. The outermost electron is one in the 3s orbital, which means that n = 3. It also means that l = 0, since s is basically shorthand for the orbital shape of l = 0. m_l can range from -l to +l, but since l = 0, then m_l must also be 0.

Relationship Between Multivalent Cation Charge Carriers And Organic Solvents On Nanoporous Carbons In 4 V Window Magnesium Ion Supercapacitors Moon 2021 Advanced Energy Materials Wiley Online Library
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next ...
What is the orbital diagram and electron configuration of magnesium? We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around ...
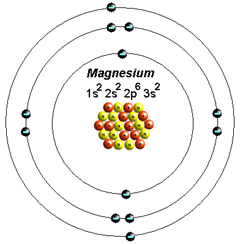
Magnesium has 2 electrons in its first shell, 8 in its second and 2 in its third. Check me out: http://www.chemistnate.com
Orbital Diagrams. • each box represents one orbital. ... electron configuration for Mg ... when it forms an ion, it loses its valence electrons from its.
Molecules Free Full Text Searching For Low Molecular Weight Seleno Compounds In Sprouts By Mass Spectrometry Html
6 Aug 2021 — The number of electrons a shell or orbital can hold is based on 'The Pauli exclusion principle. To understand any element's electron ...
The electron configuration for magnesium is 1s^2 2s^2 2p^6 3s^2. What is the distribution of electrons in the electron shells of a magnesium atom? - Get the answer to this question and access a vast question bank that is tailored for students.
An orbital box diagram puts all of the electrons of an atom in one box with their spins aligned. ... the word ion; for example Mg2+ is named magnesium ion. true. Many transition and inner transition elements form more than one positively charge ion. true. Fe3+ may be named either iron (III) ion or ferric ion ...
Lewis Structure Nitrate Polyatomic Ion Molecular Orbital Diagram Png 2418x632px Watercolor Cartoon Flower Frame Heart Download
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation

Write The Electron Configuration For Magnesium A 12 And Potassium A 19 Draw Their Orbital Brainly Ph
August 20, 2015 - Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
This video shows how to draw the orbital diagram of selenium (Se). It also shows how to write the electron configuration of selenium (Se) and the shorthand ...
How can we draw the orbital diagram of a magnesium atom and a magnesium ion. What is the orbital diagram for magnesium. 100 10 ratings or. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements. Use the buttons at the top of the tool to add sublevels. The p orbital can hold up to six electrons.
Answer to Draw an orbital diagram for each element: (a) magnesium; (b) aluminum; (c) bromine..

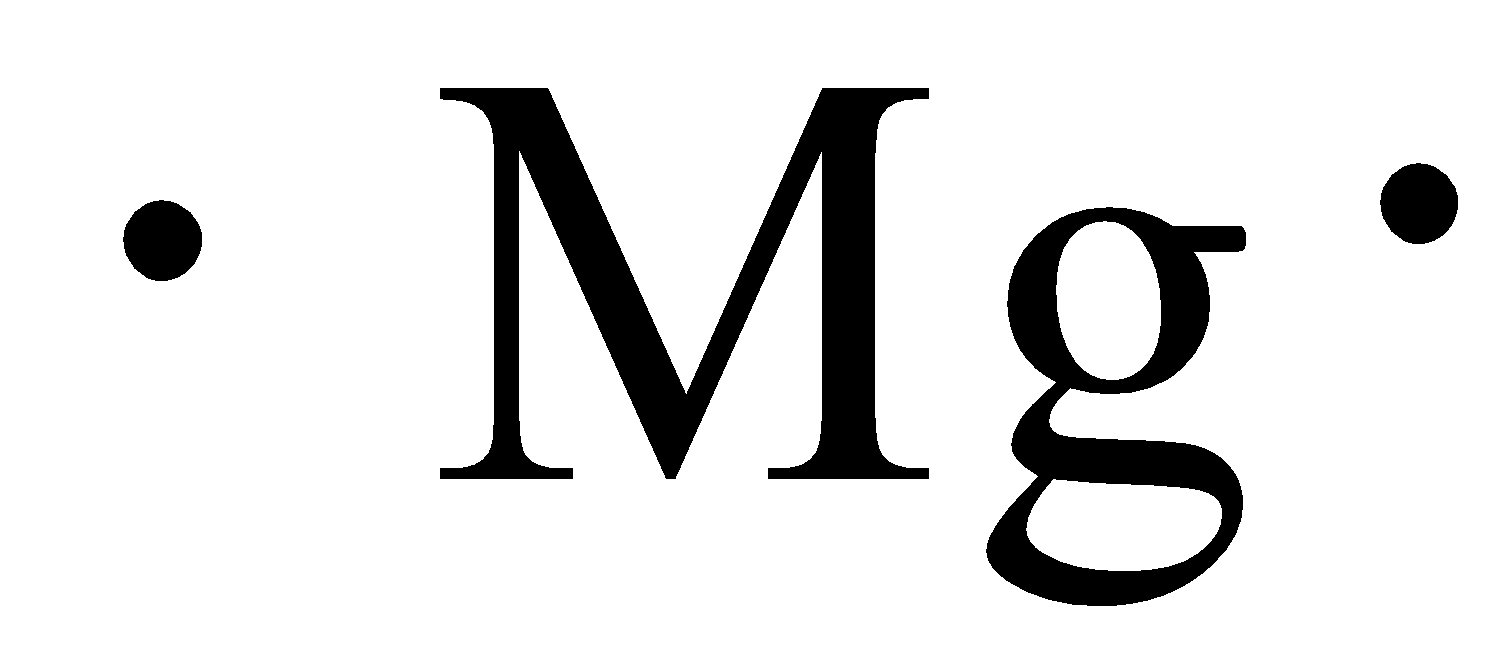






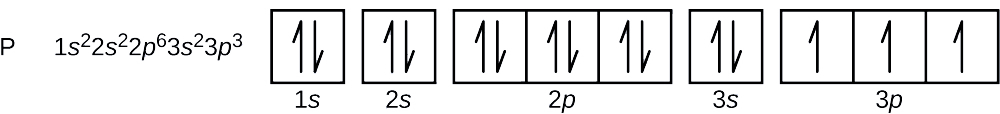








0 Response to "36 orbital diagram for magnesium ion"
Post a Comment