35 bohr diagram for carbon
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Beside above, what is the Bohr diagram for hydrogen?
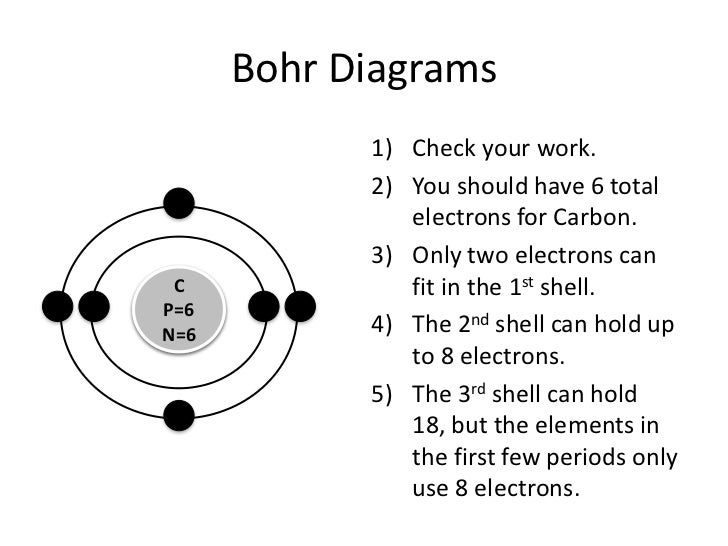
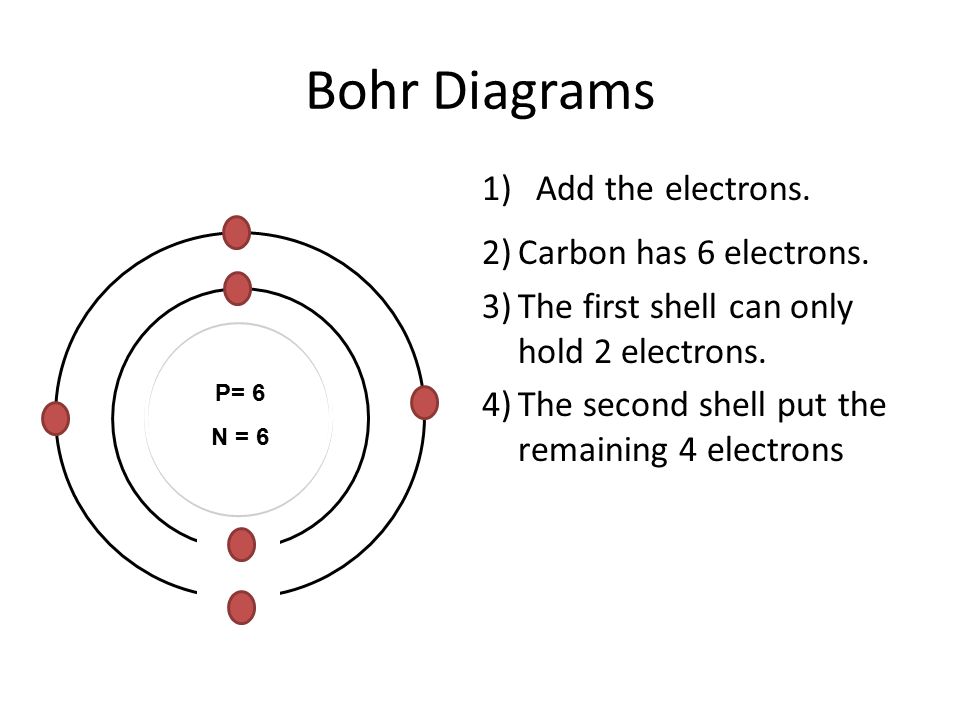
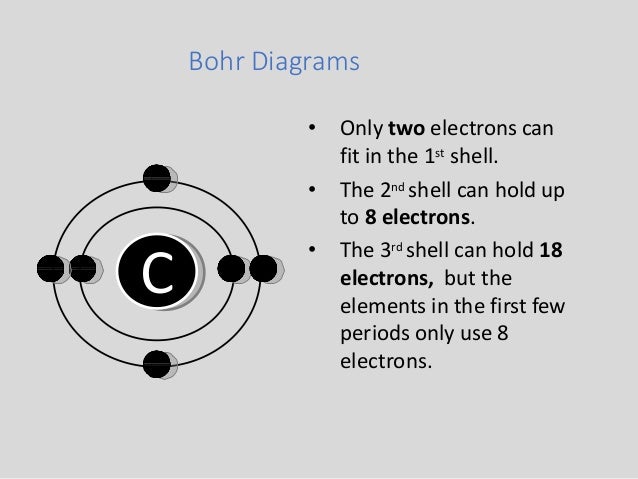
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st energy level. 4) The 2nd energy level can hold up to 8 electrons. 5) The 3rd energy level can hold 18, but the outer shell can only hold 8 electrons. C P+ = 6 N0 = 6 2e-4e-
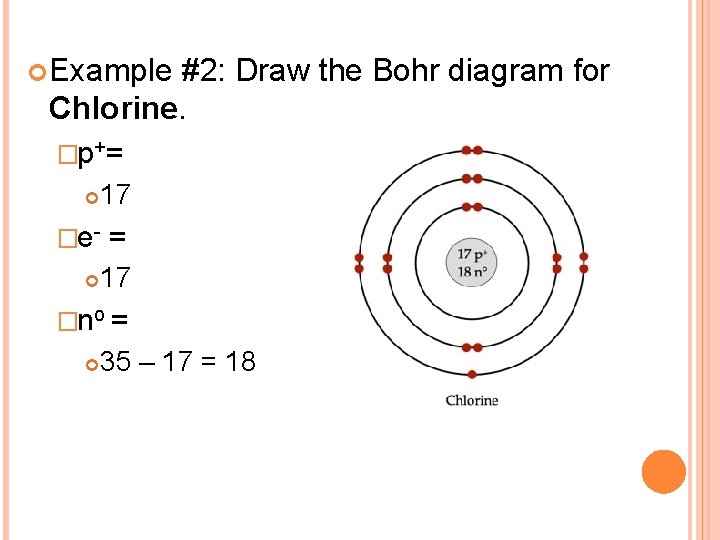
Bohr's diagram of Chlorine has three electron shells (K, L, and M), the inner shell is K-shell and the outermost shell is M-shell. Hence, the electrons found in the M-shell of the Chlorine atom are its valence electrons because it is the outermost shell that also called the valence shell.

Bohr diagram for carbon
In atomic physics, the Bohr model or Rutherford-Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the solar system Joseph Larmor model (1897), the cubical model (1902 ...
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.
From the Bohr diagram of an atom, we can easily find the number of valence electrons in an atom by looking at its outermost shell. Let's take the example of the Neon Bohr model. Bohr's diagram of Neon has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell.
Bohr diagram for carbon.
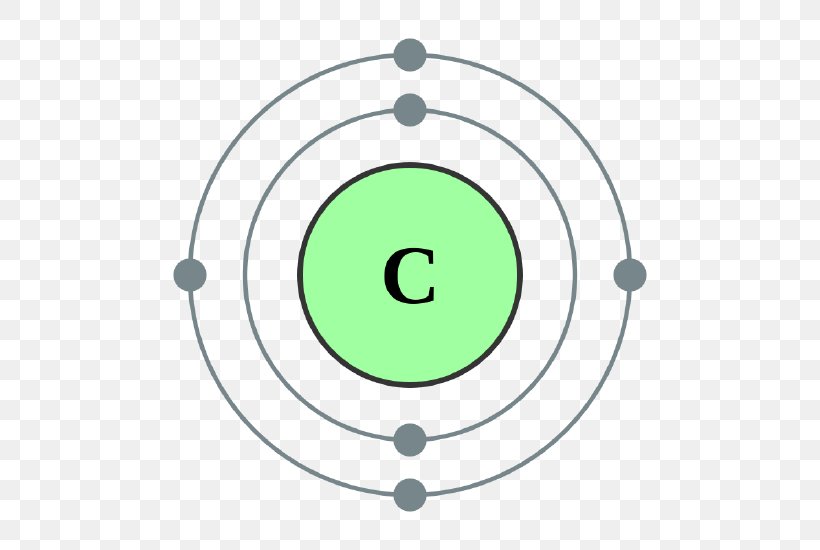
Carbon has 2 electrons in its first shell and 4 in its second shell.Check me out: http://www.chemistnate.com
Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out: http://www.chemistnate.com
The Bohr Model of Phosphorus(P) has a nucleus that contains 16 neutrons and 15 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Phosphorus contains 5 electrons that also called valence electrons.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
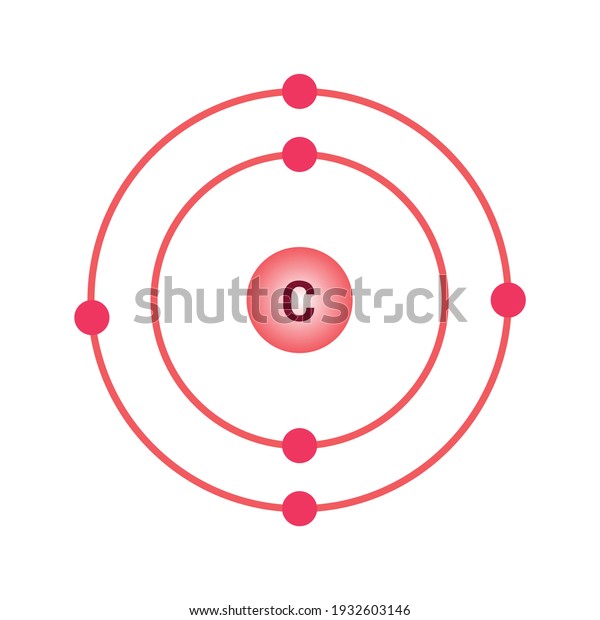
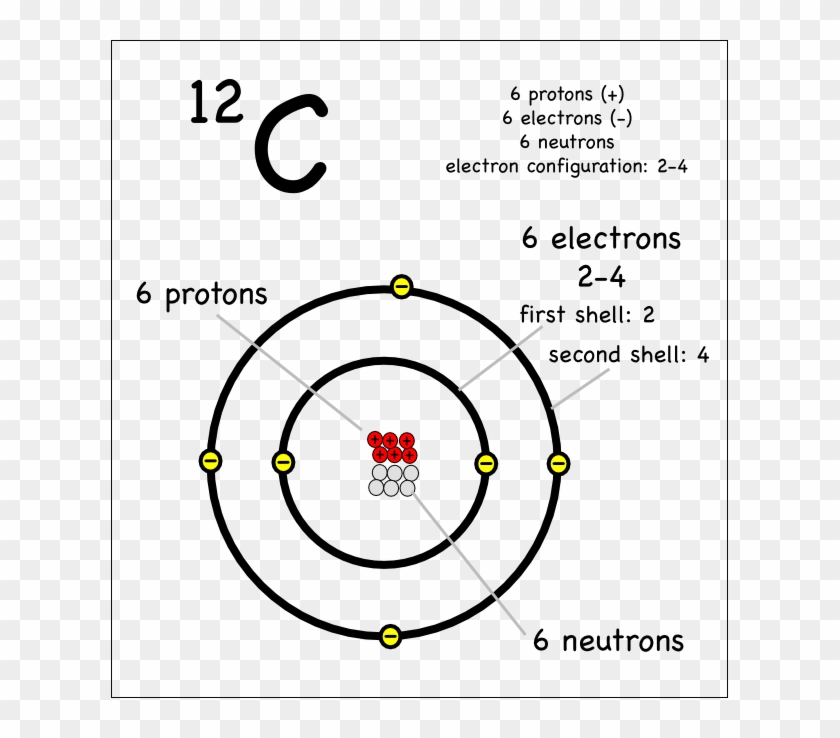
Bohr's diagram of Carbon has only two electron shells (K and L), the inner shell is K-shell and the outermost shell is L-shell. Hence, the electrons found in the L-shell of the Carbon atom are its valence electrons because it is the outermost shell that also called the valence shell.
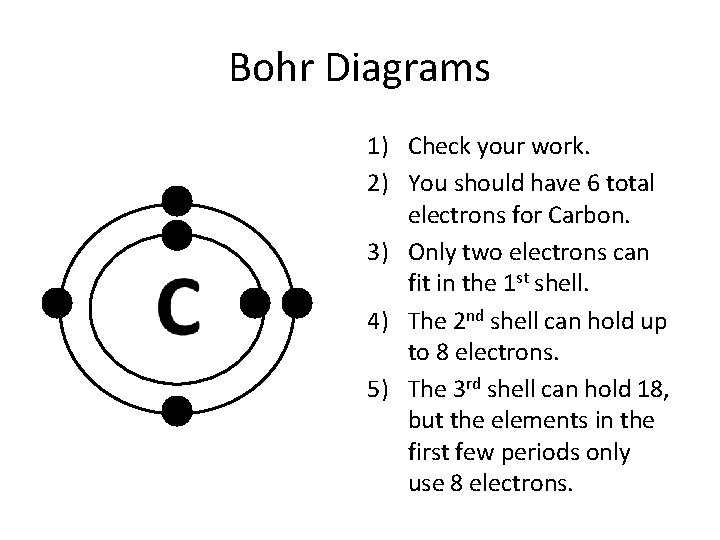
Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. The 2nd shell can hold up to 8 electrons. This is answered comprehensively here. Considering this, what is the Bohr diagram for carbon? Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons.
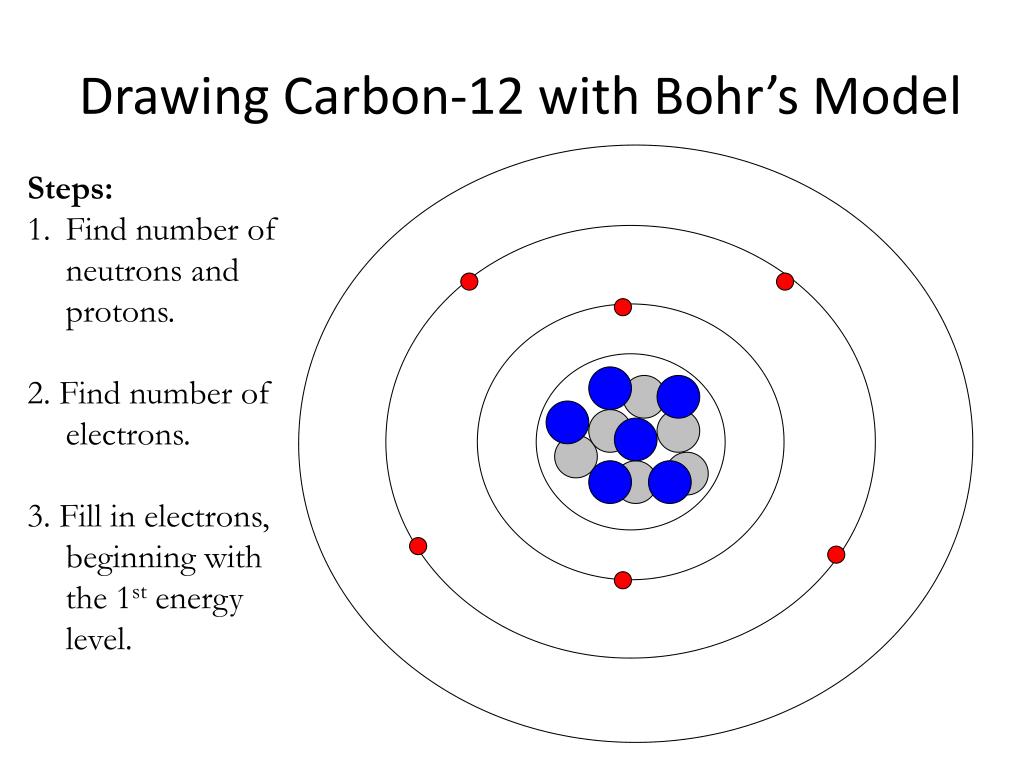
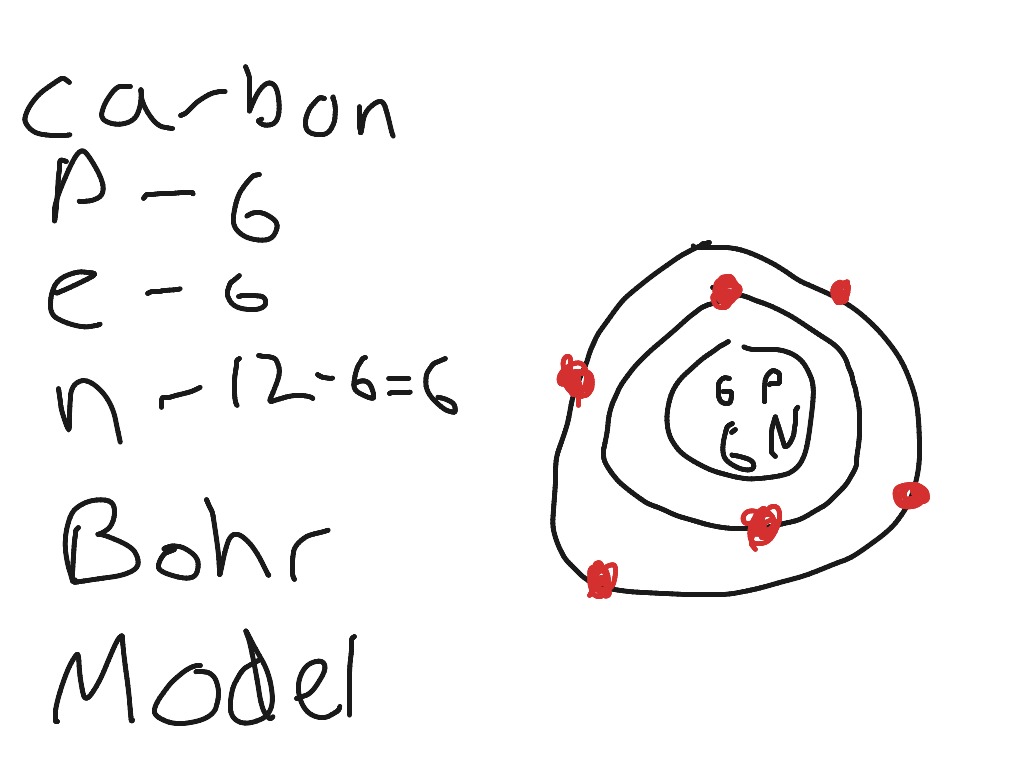
To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. ... For example, carbon has an atomic number of 6 and an atomic weight of 12. So a ...
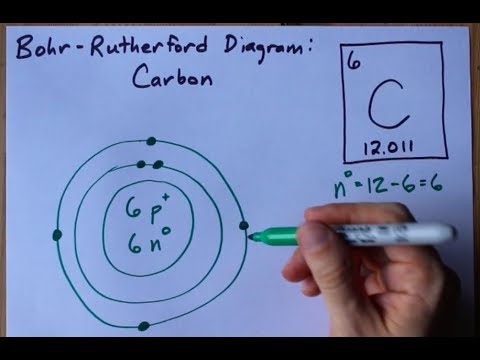
8. <ul><li>You now have a complete Bohr Diagram </li></ul>Bohr Diagrams Carbon Atomic # = 6 Mass # = 12 Atomic mass = 12.011 amu C 6p + 6n 0 9.
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
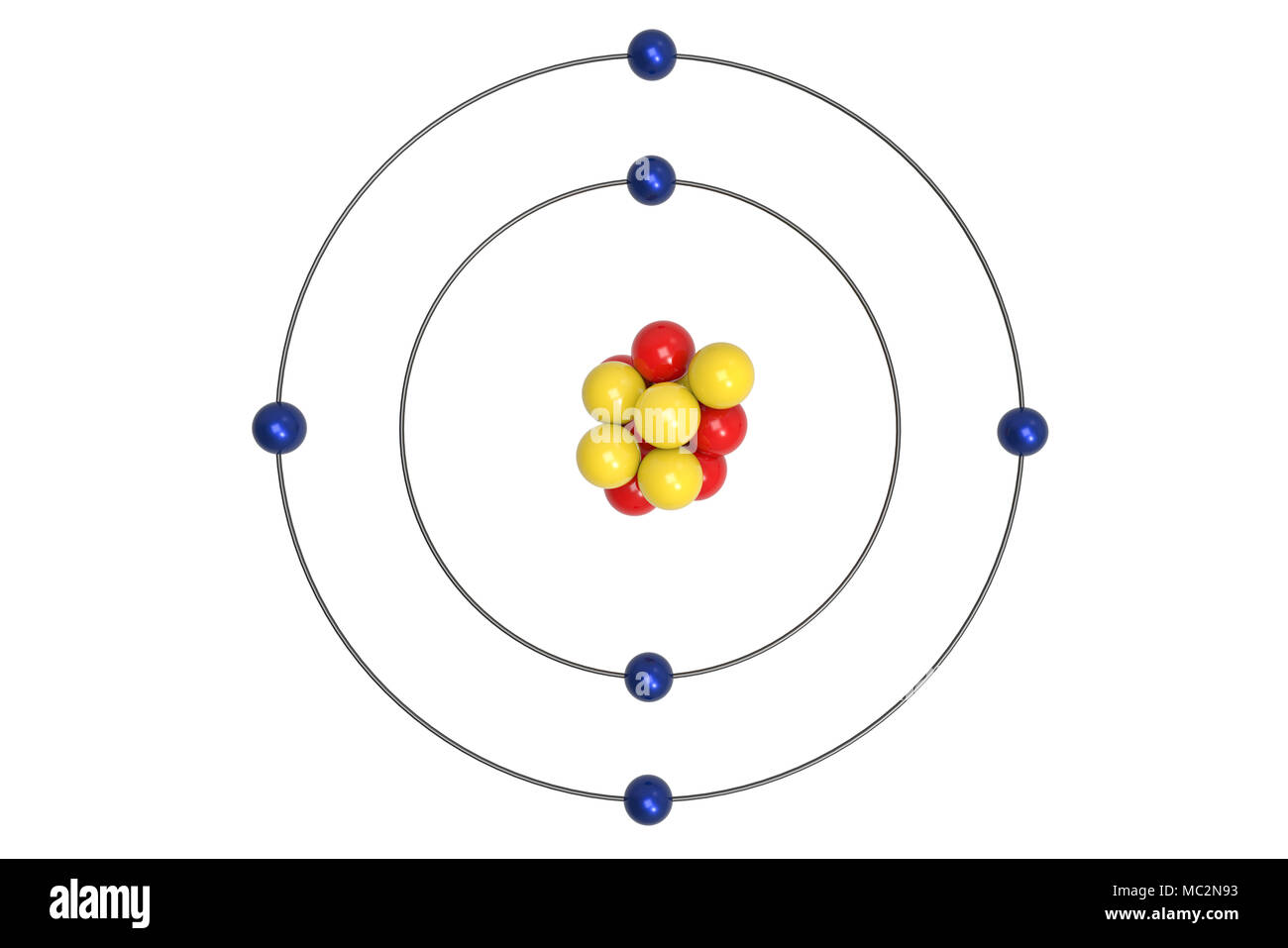
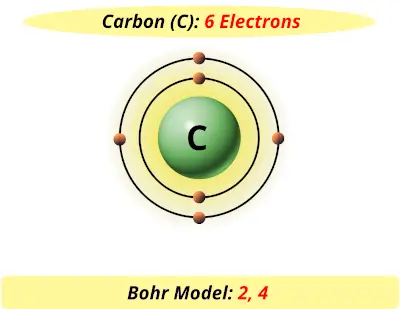
The Bohr model of carbon has a central nucleus containing six protons and six neutrons, encircled by an inner orbit of two electrons and an outer orbit of four electrons. The two orbits represent different energy levels and are at a set distance from one another and from the nucleus. In the Bohr model, electrons with less energy occupy orbits ...
Carbon-12 consists of six protons and six neutrons (Figure 4). Most naturally occur-ring carbon has this atomic structure. Th ere is, however, another form of carbon called carbon-14. Carbon-14 has six protons and eight neutrons. Carbon-14 is a dif-ferent isotope than carbon-12. Diff erent isotopes of an element have the same number
What is the Bohr model for carbon? A Bohr model is a way of visually depicting the structure of an atom, specifically to show the location of its electrons in their energy levels. The Bohr model shows that the protons and neutrons are located in the nucleus, while electrons orbit in energy levels. Also question is, what does Bohr's model explain?
Carbon Dioxide Bohr Diagram. The Bohr effect is a physiological phenomenon first described in by the Danish Conversely, a decrease in carbon dioxide provokes an increase in pH, which His proposed model was flawed, and Bohr harshly criticized it in his own . Sep 9, Bohr diagram of carbon dioxide.
A Bohr model is a way of visually depicting the structure of an atom, specifically to show the location of its electrons in their energy levels. An atom is the smallest component of matter and ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n
Sep 24, · For the Carbon Dioxide Lewis structure, calculate the total number of valence electrons for the Carbon Dioxide molecule (Carbon Dioxide has 16 valence electrons). That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon dioxide or the pH of the environment.
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen-haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon ...
The Bohr Model of Sulfur(S) has a nucleus that contains 16 neutrons and 16 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sulfur contains 6 electrons that also called valence electrons.
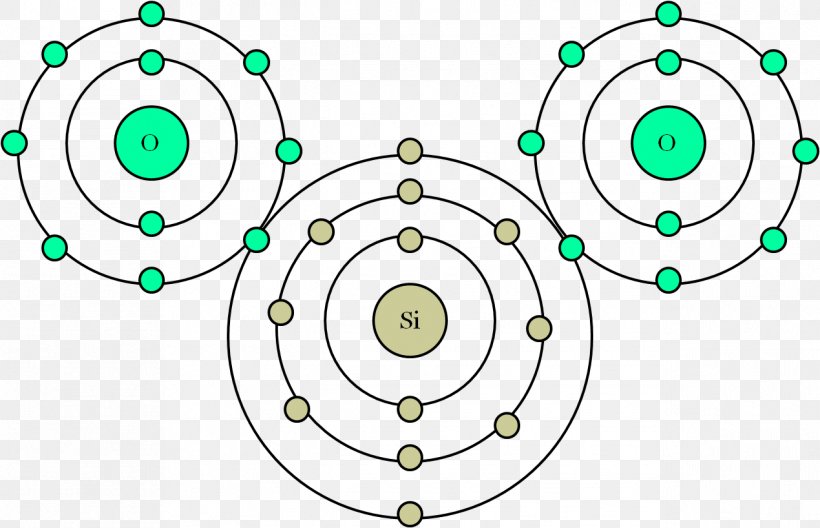
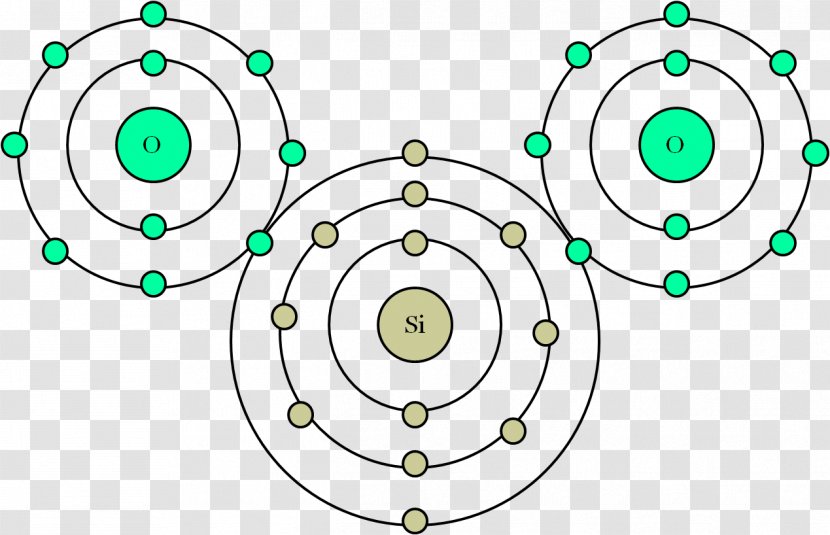
Carbon is a non-metal which has 4 valence electrons (that means 4 electrons in its outer shell, the second).Oxygen is also a non-metal, with 6 electrons in i...
HOW TO DRAW BOHR DIAGRAMS - In this video, I'll teach you how to draw bohr diagrams for carbon (C), sodium (Na), and phosphorous (P). The steps to drawing bo...
Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer. Last class, we determined that the Bohr Model is a planetary model in which the For example, there are 3 shells in the bohr diagram of Argon.
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...














0 Response to "35 bohr diagram for carbon"
Post a Comment