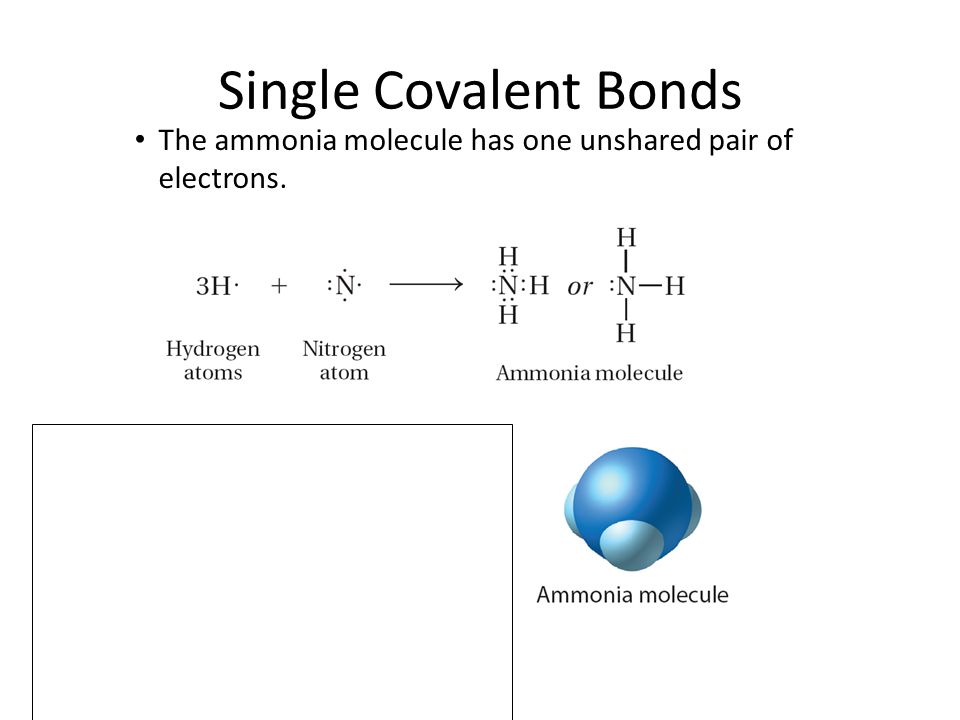
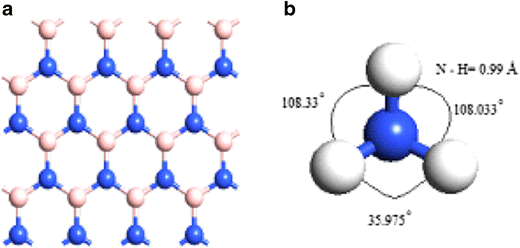
36 the ammonia molecule in the diagram has the observed bond orientation because ...
1. By making two covalent bonds, an O atom with 8 protons fills its valence shell. why does the atoms charge stay close to zero?a. The atom lost electrons from other; Question: 1. By making two covalent bonds, an O atom with 8 protons fills its valence shell. why does the atoms charge stay close to zero?a. The atom lost electrons from other
In a double covalent bond, a carbon atom shares ... electrons in two orbitals. The ammonia molecule in the diagram has the observed bond orientation because ...
The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above

The ammonia molecule in the diagram has the observed bond orientation because ...
The ammonia molecule in the diagram has the observed bond orientation because 30 more tips 1994 ford ranger fuse box diagram 30 even more tips troy bilt pony parts diagram 50 more step and image. Chap 2 Chemical Bond Chemical Polarity None of the above. The ammonia molecule in the diagram has the observed bond orientation because. Each pair of ...
The ammonia molecule in the diagram has the observed bond orientation because ... a. N has four pairs of electrons in the valence shell
The ammonia molecule in the diagram has the observed bond orientation because ... All of the above. electrons repel one another. N has 7 protons in its ... Rating: 4,9 · 18 reviews
The ammonia molecule in the diagram has the observed bond orientation because ....
Covalent Bonds hold atoms together because they. ... The ammonia molecule in the diagram has the observed bond orientation because. Rating: 4,9 · 15 reviews
The ammonia molecule in the diagram has the observed bond orientation because. N has four pairs of electrons in the valence shell b. The electrons form 3 bonds and 1 lone pair of electrons. All of the above. The ammonia molecule in the diagram has the observed bond orientation because. Electrons repel one another c. N has 7 protons in its nucleus.
The ammonia molecule in the diagram has the observed bond orientation because. The ammonia molecule in the diagram has the observed bond orientation because. N has four pairs of electrons in the valence shellb. Electrons repel one another c. Rotation can occur around single bonds. All of the above e. N has 7 protons in its nucleus.
compared with 31P, the radioactive isotope 32P has? ... the atom has more electrons than protons ... Covalent bonds hold atoms together because they. Rating: 4,5 · 2 reviews
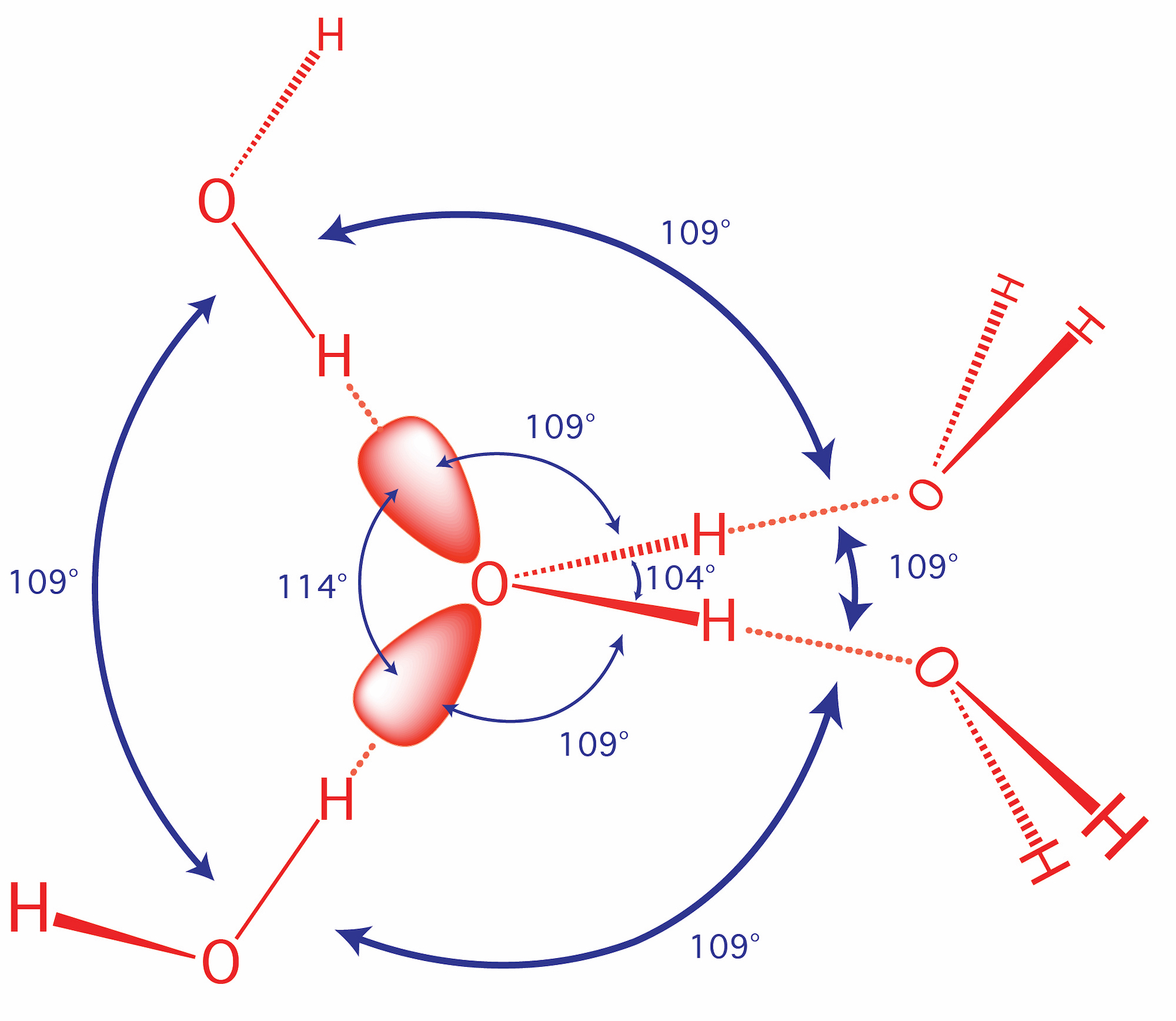
Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR ...
View full document. The ammonia molecule in the diagram has the observed bond orientation because…. All of the above (N has 7 protons in its nucleus, electrons repel one another, N has four pairs of electrons in the valence shell) The discovery of which of the following led to a drastic change in deep sea mining?
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.
The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field.
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.




















0 Response to "36 the ammonia molecule in the diagram has the observed bond orientation because ..."
Post a Comment