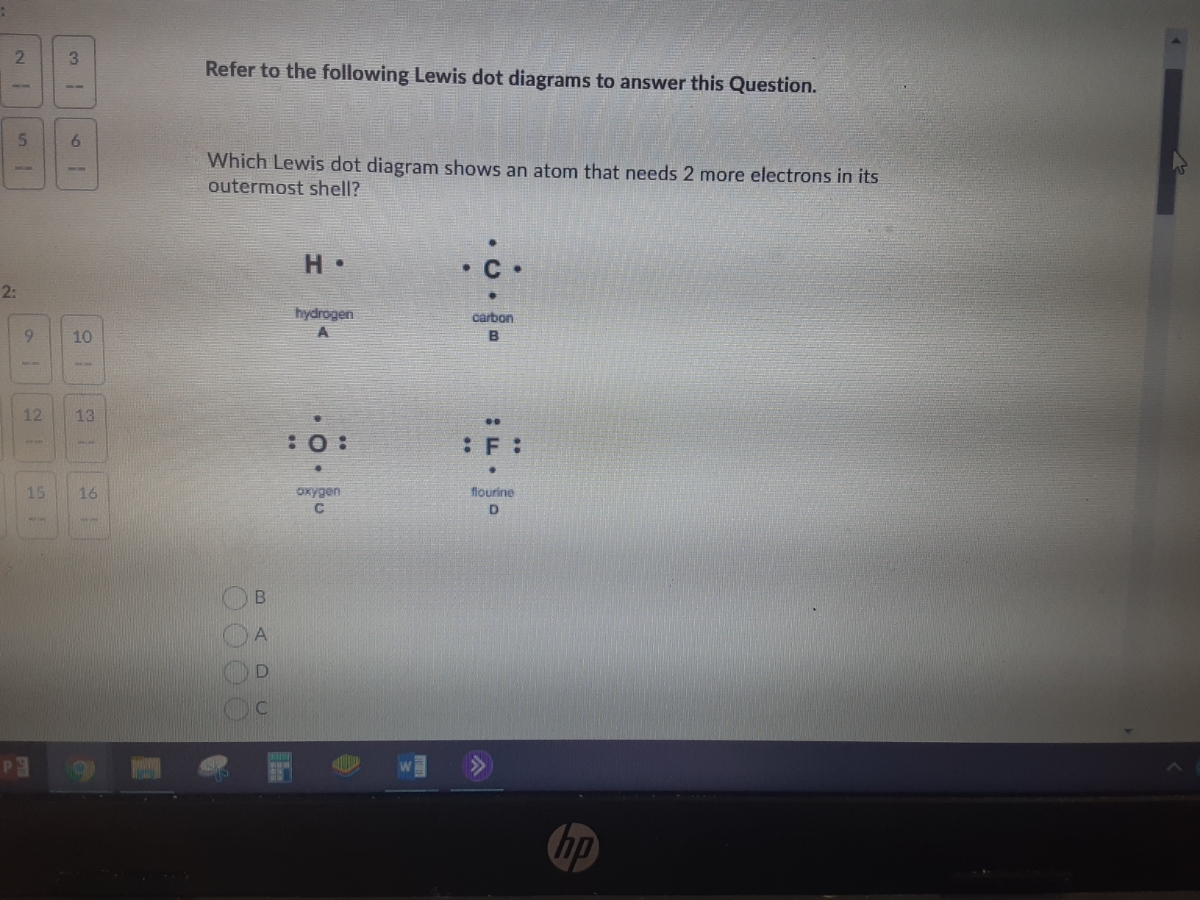
37 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?
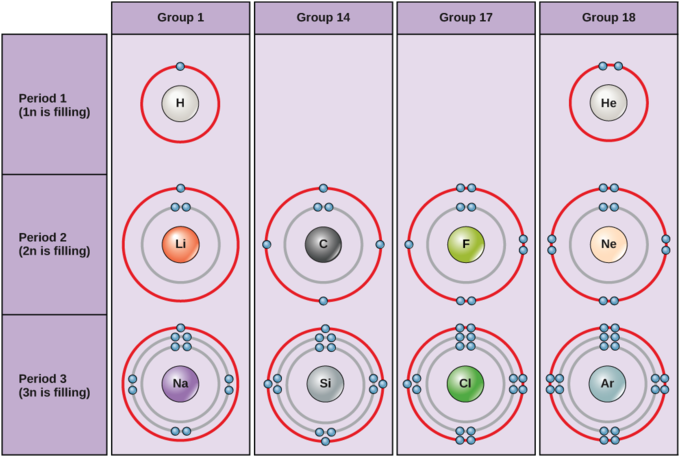
The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons.
Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? H • hydrogen carbon A :0: :F : oxygen flourine C B Refer to the following Lewis dot diagrams to answer this Question.
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?
30 Questions Show answers. Q. How many electrons should Oxygen have around its Lewis dot model? Which of the following shows a correct Lewis dot structure? Which element could X represent? Q. How many electrons should Carbon have around its Lewis dot model? Q. According to the octet rule most elements need _______ valence electrons.
In a lewis dot diagram, each element will appear to have 8 val…. Lone pair of electrons. A pair (2) of electrons not involved in bonding. double bond. sharing of 4 electrons between 2 atoms. 84 Terms. A_Jimenez56. Unit 2 - Bonding, Nomenclature, Lewis Dot Diagrams, and Molecular Shape. Ammonium.
A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?.
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
Answer: Well, we push electrons around, and distribute them as pairs…and given a molecule, say NO_{2}, we first must decide how many VALENCE electrons we got to start. And for oxygen, Z=8, and nitrogen, Z=7, and 6, and 5 valence electrons with which to play…the other 2 electrons, formally the 1s^...
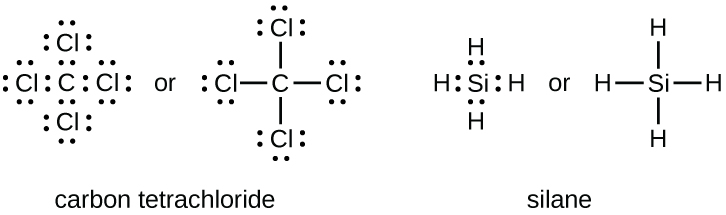
Carbon has four valence electrons and it needs four more electrons to complete eight electrons to its valence shell. So, in Lewis dot structure of the carbon atom is the central atom and two H and two F atoms are bonded to it with single covalent bonds.. The molecular geometry of the molecule is tetrahedral(in box form).
Play this game to review Chemistry. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Preview this quiz on Quizizz. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Unit 7 Test Review DRAFT. 10th grade. 11 times. Chemistry. 63% average accuracy. 5 months ago ...
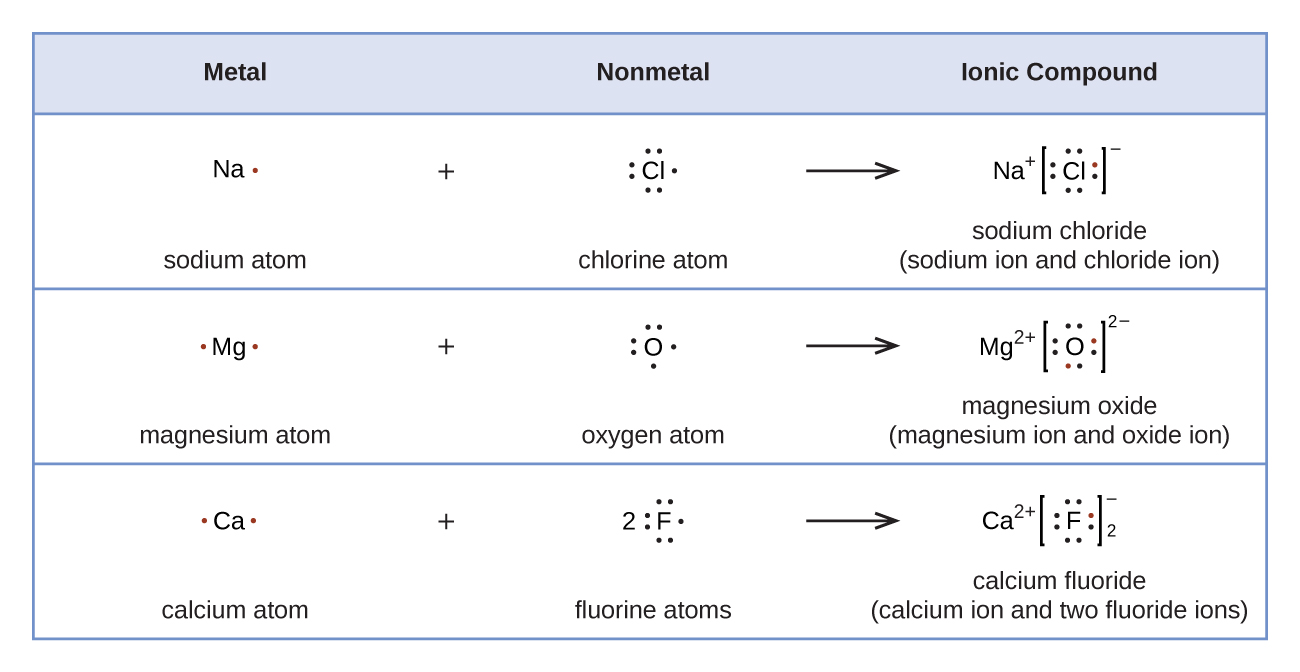
2 CHEM 1411. Chapter 7. Chemical Bonding I (homework) W ____ 6. Calcium and chlorine react to form CaCl 2, an ionic compound. The chloride ion, Cl , has ____ electrons in its outermost occupied shell.
The correct electron-dot formulation for hydrogen cyanide shows: (a) 2 double bonds and two lone pairs of electrons on the N atom. (b) 1 C-H bond, 1 C=N bond, 1 lone pair of electrons on the C atom and 1 lone pair of electrons on the N atom. (c) 1 C-H bond, 1 C-N bond, 2 lone pairs of electrons on the C atom and 3 lone pairs of electrons on the ...
Lewis Electron Dot Structure of Ethane, C 2 H 6. The electronic configuration of carbon atom shows that it needs four more electrons to fill up its outer shell; the hydrogen atom, on the other hand, needs just one more electron for its lone electron shell.
39 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell Written By Rosa B. Pruitt. Thursday, December 2, 2021 Add Comment Edit. ... Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell. Lewis symbols ...
Rules for Writing Lewis Structures. Count the total number of valence electrons in the molecule or polyatomic ion. (For example, H 2 O has 2x1 + 6 = 8 valence electrons, CCl 4 has 4 + 4x7 = 32 valence electrons.) For anions, add one valence electron for each unit of negative charge; for cations, subtract one electron for each unit of positive charge.
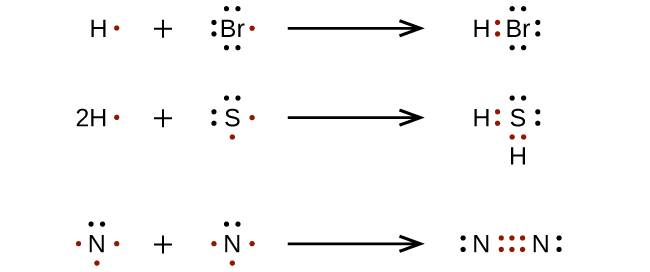
Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. You can draw a Lewis dot structure for any covalent molecule or coordination ...
Lewis Diagram (Electron Dot) In most stable molecules or polyatomic ions, each atom tends to acquire a noble-gas structure by sharing electrons. This tendency is often referred to as the octet rule. One way to show the structure of an atom or a molecule is using dots to represent the outermost s-and p-electrons (the so-called valence electrons).
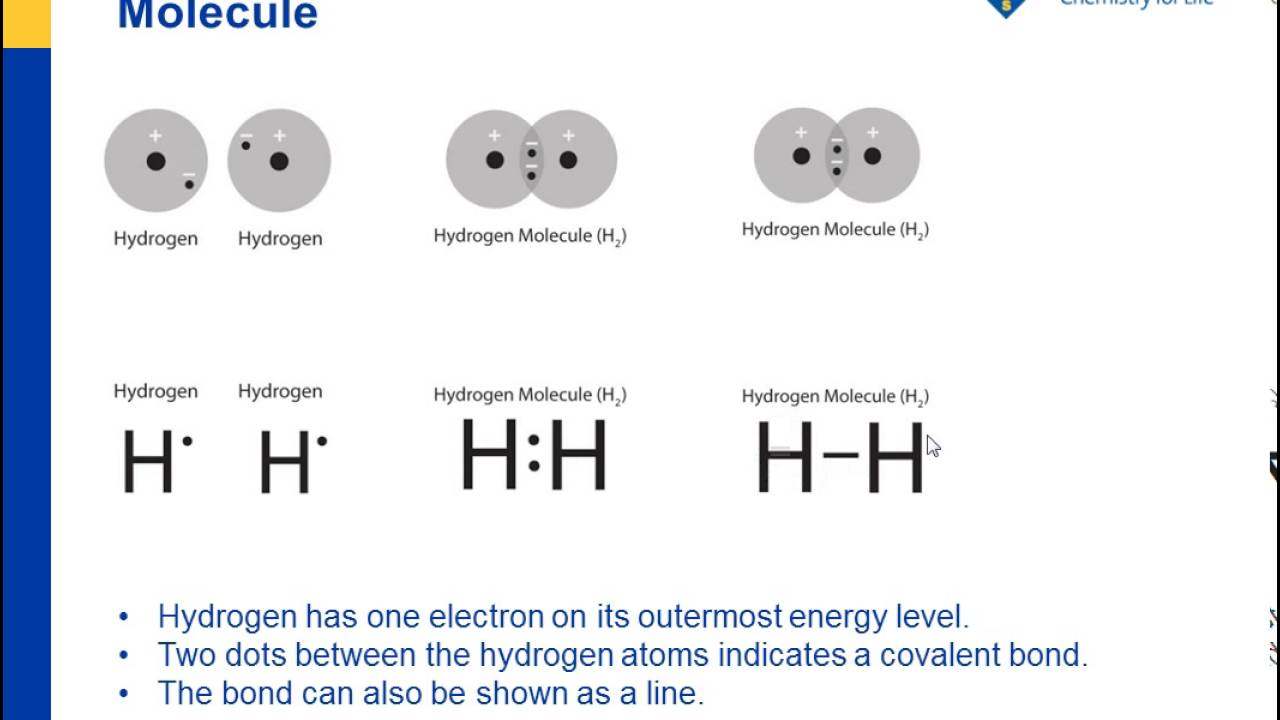
To write a Lewis symbol for an atom, place the atom's chemical symbol in the center. This symbol will be the atomic core and will represent the nucleus and inner electrons for that atom. The valence electrons will be represented as a dot. Dots are placed around the atomic core singly for the first four electrons.
For period 2 elements, where all the valence electrons of an atom are in s and p orbitals, we find that the Lewis dot structure of molecules will often follow the Octet Rule:. Octet Rule - Atoms tend to gain, lose, or share electrons until they are surrounded by eight electrons (4 electron pairs).. Using Lewis dot structures and the octet rule, we can predict and represent the electronic ...
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
Valence shell is 2s 2 2p 3 with total 5 electrons. Lewis dot structure of N atom. Let's do one more example: Se atom. Electronic configuration: [Ar]3d 10 4s 2 4p 4. Valence shell is 4s 2 4p 4 with total 6 electrons. Lewis dot structure of Se atom Lewis dot structure of Cl atom Lewis dot structure of all atoms of the main periodic table
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
An atom may hold maximum 8 electrons in its outermost shell 2 in 's' orbital and 6 in 'p' orbitals, the next incoming electron enters in next energy level because next 's' orbital fills prior to ...
Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? C Which type of energy is the energy that it takes to remove an electron from its shell?
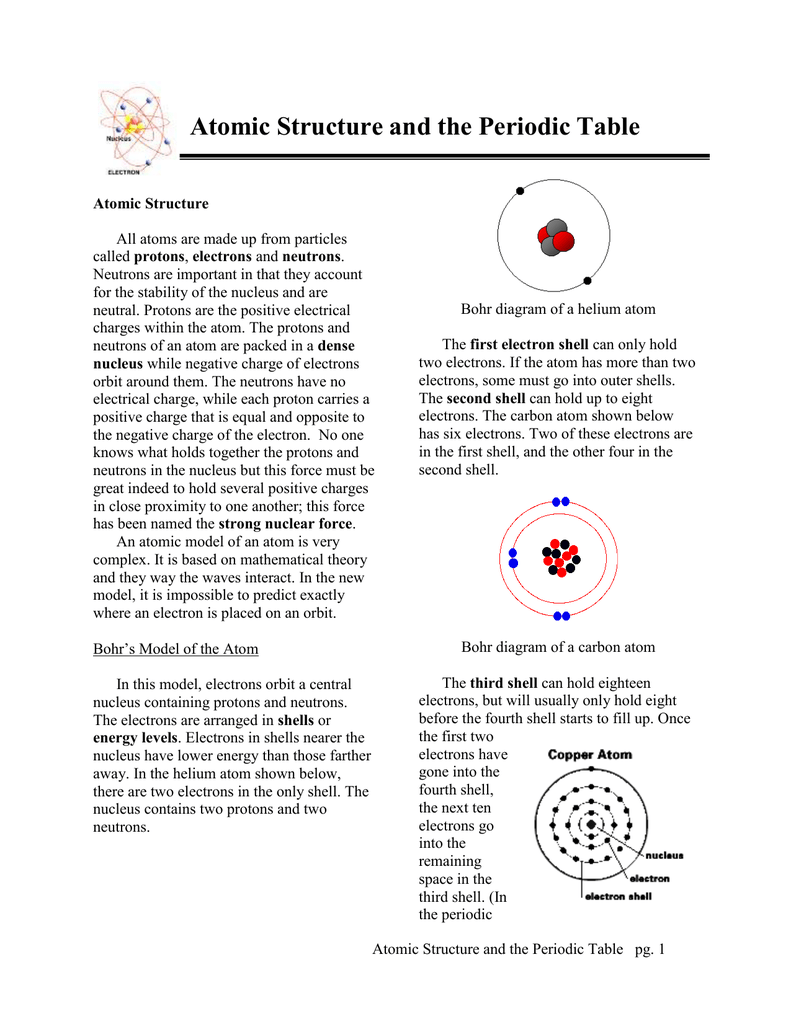
2. Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
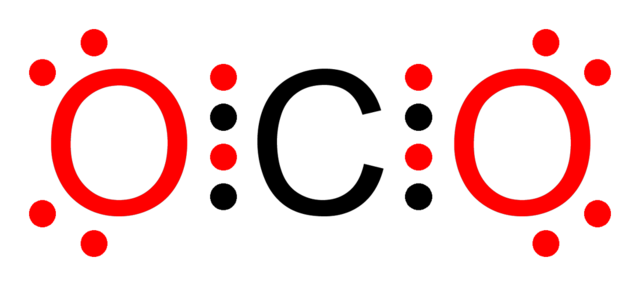
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. There are also two more valence electrons and they are paired ...
Answer (1 of 2): You have got three atoms, first choose which one will be the central, and that is Oxygen. Now draw separately the atomic diagram of each atom so that you would know that how many valence electrons are in each one of them. Now you know that Oxygen has 6 electrons in its valence ...
In a Lewis dot diagram, the electrons shown: c) Are in the outermost energy level.. A Lewis dot diagram (structure) can be defined as a structural representation of the atom or molecule of a chemical element in an atomic orbital, by using a dot to show the position and distribution of electron(s) around the atom or molecule.. This ultimately implies that, a Lewis dot diagram is typically used ...





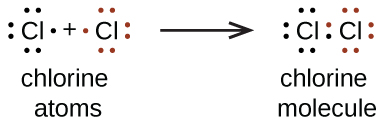
![Best Answer] Choose the write Lewis electron dot diagram for ...](https://us-static.z-dn.net/files/d88/47b81ffdeb97e849146b837bc52b2301.png)





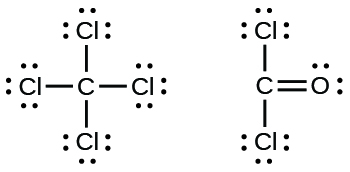








0 Response to "37 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?"
Post a Comment