39 co molecular orbital diagram bond order
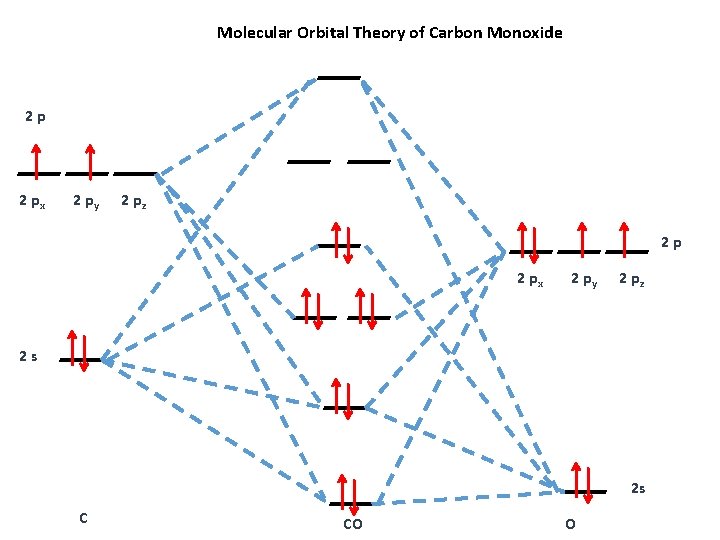
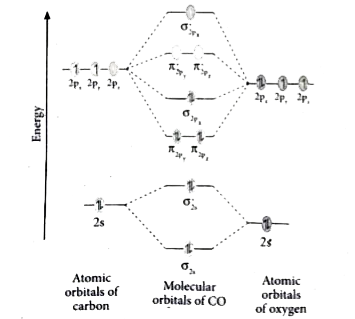
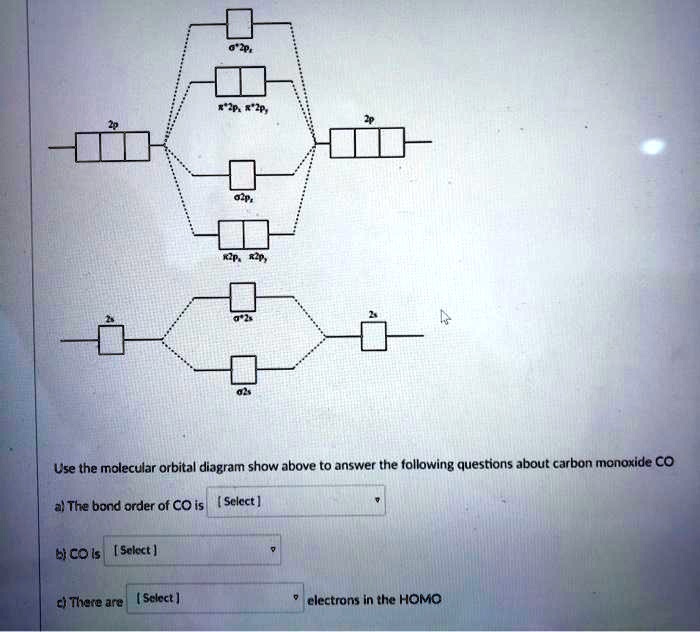
CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as. σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰. Thus , bond order = 1/2(8–2)=3
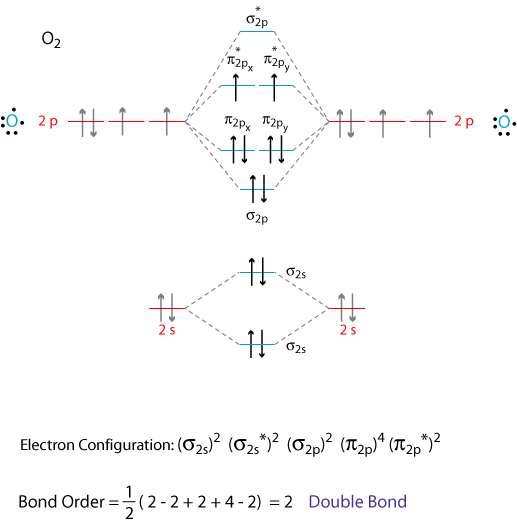
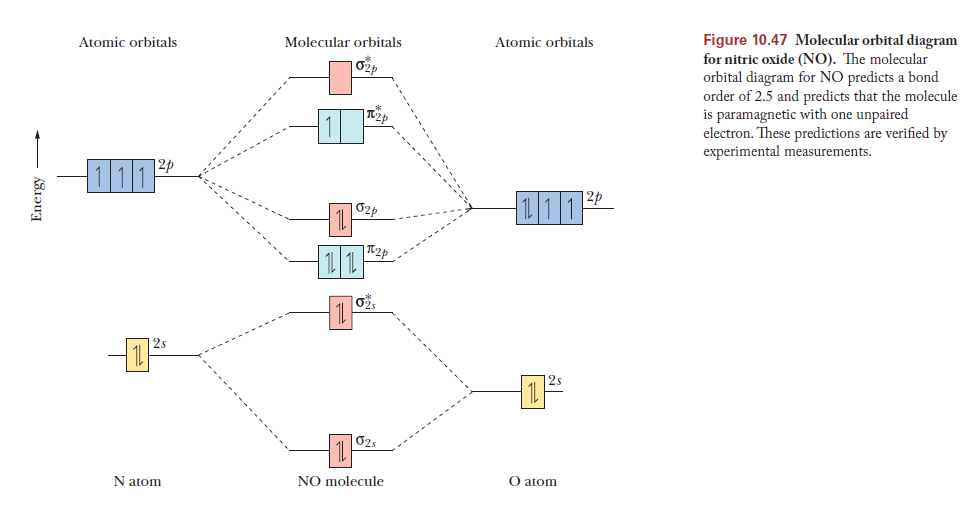
"O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.
Orbital-orbital Interactions and Symmetry Adapted Linear Combinations; ... Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules.

Co molecular orbital diagram bond order
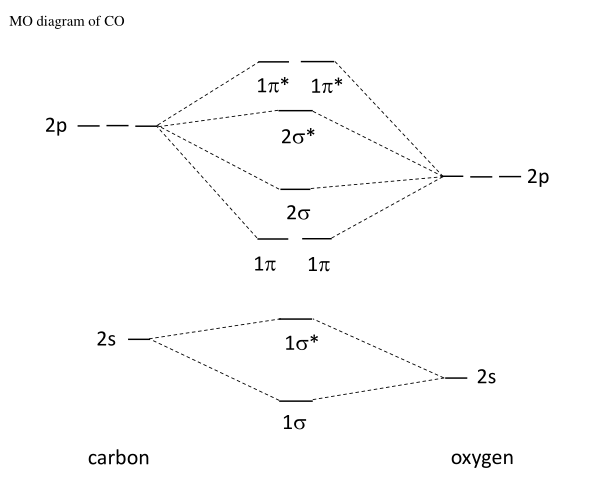
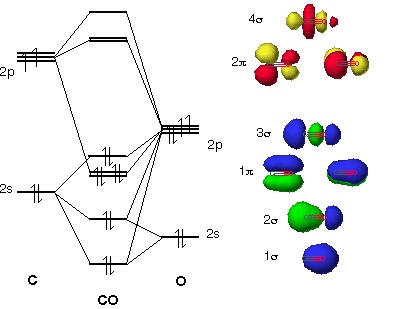
Bonding in some hetero nuclear di-atomic molecules: Molecular orbital diagram of Carbon monoxide molecule (CO). Electronic configuration of C atom: 1s2 2s2 ...
CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen atom (2s²2p⁴).According to molecular orbital diagram, molecular orbital configuration is given as. σ2s² σ*2s² πx² πy² σz² π*x⁰ πy⁰ σ*z⁰. Thus , bond order = 1/2(8–2)=3
Bond Order. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the filling of the molecular orbitals by electrons.
Co molecular orbital diagram bond order.
Higher is the bond order greater is the stability of molecule. i) If Nb > Na, then molecule ... Molecular Orbital diagram of Carbon monoxide molecule (CO):.
The molecular orbital diagram of carbon monoxide, CO, is show below. Which overlap is strongest? During the axial overlap of p-p orbitals, the electron density increases around the axis, so the bond formed is the strongest.
Carbon monoxide MO diagram — Carbon monoxide MO diagram. Carbon monoxide is an example of a heteronuclear diatomic molecule where both atoms are second-row ...Molecular orbital diagrams for... · Carbon monoxide MO diagram
Calculate a molecule's bond order given its molecular orbital diagram. ... In carbon monoxide (CO), the oxygen 2s orbital is much lower in energy than the ...
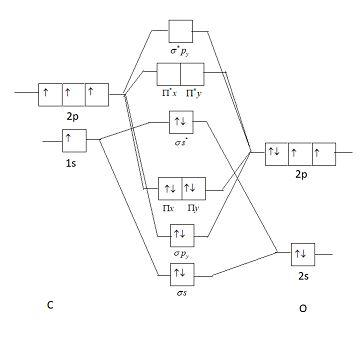
Bond order=1/2 (bonding−anti-bonding) According to molecular orbital diagram, the bond order of CO+ is 3.5. The highest occupied molecular orbital is sigma*2s MO. In the case oc CO, the 2s atomic orbital on oxygen is much lower than the energy than the 2s atomic orbital of carbon. This discrepancy of energy allows the pi2px & pi2py BMO to ...
4) or carbon dioxide (CO 2), a MO diagram may show one of the identical bonds to the central atom. For other polyatomic molecules, an MO diagram may show ...Basics · s-p mixing · Diatomic MO diagrams · Heteronuclear diatomics
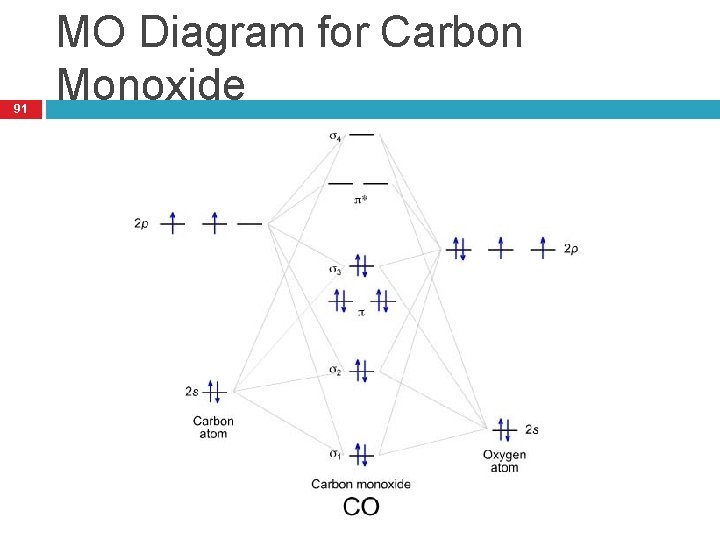
Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ...
The bond order of CO is 3 as oxygen shares two of its electrons with carbon and makes a dative bond filling it’s shell. You can also simply prove it by molecular orbital theory. What is the bond order of hydrogen fluoride? one In hydrogen fluoride (HF), the hydrogen 1s orbital can mix with the fluorine 2pz orbital to form a sigma bond because ...
Molecular orbital energy level diagram of CO molecule can be given as. · CO molecule has 10 valence electrons,four from carbon atom (2s²2p²) and six from oxygen ...10 answers · 9 votes: bond order = 3 Explanation = (1) no of electron in C = 6 (2) no of electron in O = 8 So, ...
The bond order of CO molecule on the basis of molecular orbital theory is: (1) Zero (2) 2 (3) 3 (4) 1 ... The bond order shows the number of chemical bonds ...1 answer · Top answer: Answer: (3) The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference ...


















0 Response to "39 co molecular orbital diagram bond order"
Post a Comment