37 orbital diagram for neon
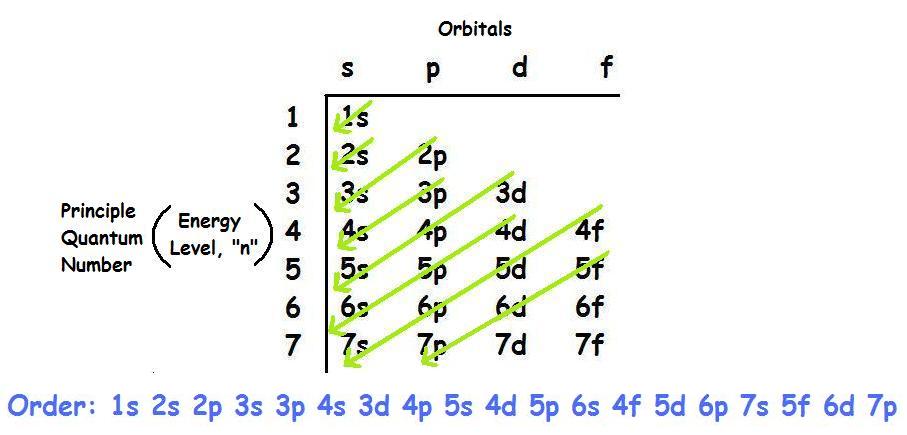
Orbital diagrams and electron configurations Pre-AP (1 ... 4) Orbital Diagram: Orbitals are sometimes shown as boxes in a horizontal row Remember: s = 1 orbital p = 3 orbitals d = 5 orbitals f = 7 orbitals 5) Arrows are used to represent the electrons , so if two arrows go in the same box, one points up and the other points down. Molecular Orbital Diagram of Neon Molecule - Nature of ... Molecular Orbital Diagram of Neon Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Wat...
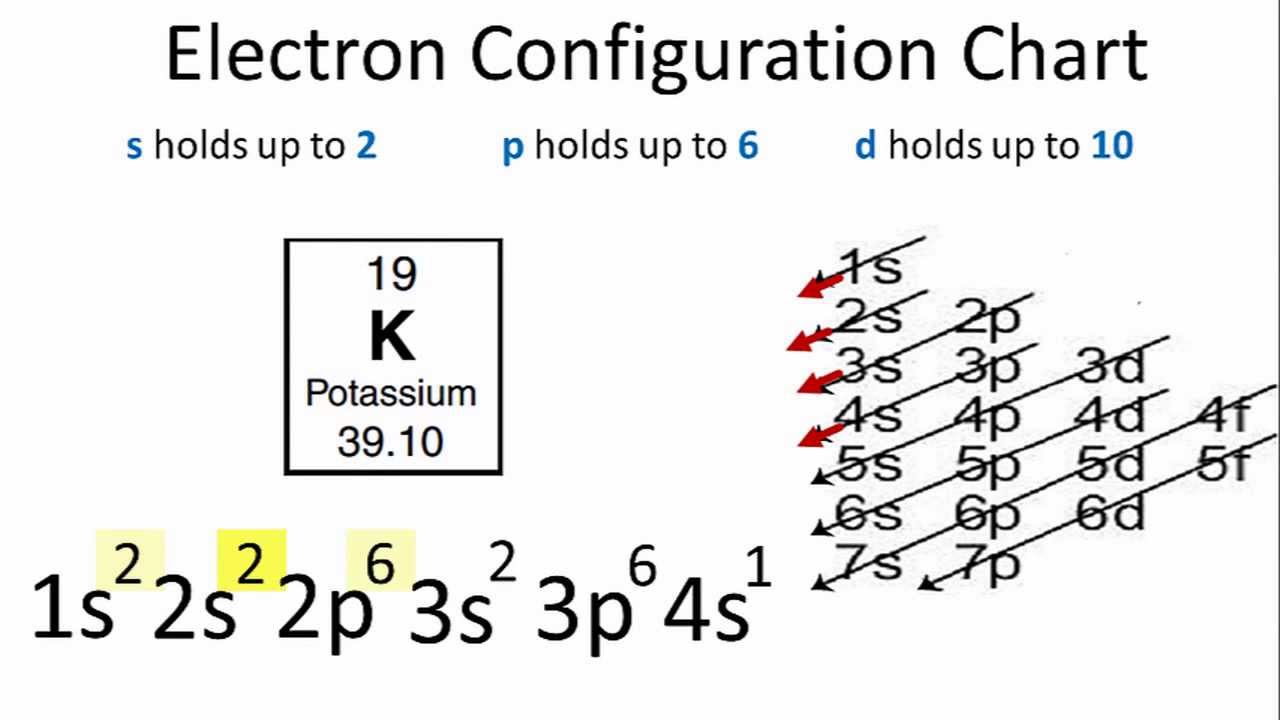
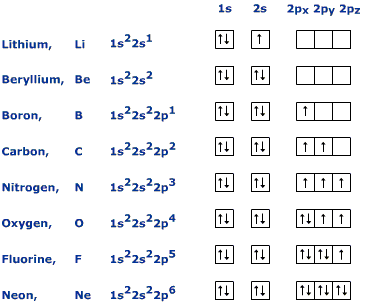
Electron Configurations and Orbital Box Diagrams ... An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
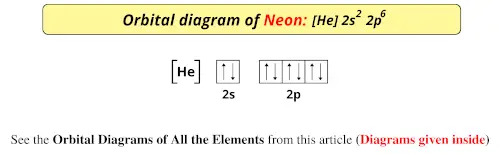
Orbital diagram for neon
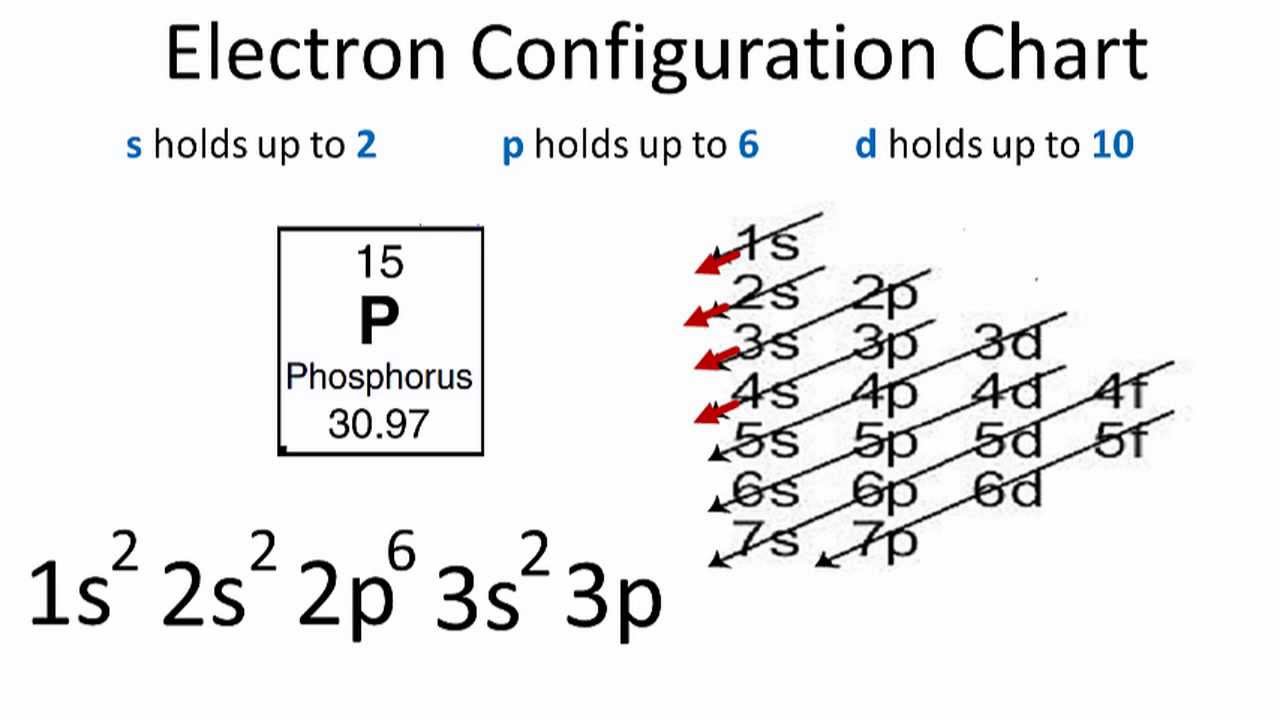
What is the electron configuration of the neon atom ... [2,8] or 1s^2 2s^2 2p^6 Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. The n=1 shell can only hold 2 electrons, so the remaining 8 electrons fill the n=2 shell. The more detailed version of the electronic configuration reflects the sub-shells in the n=1 and n=2 shells, where the n=1 shell just has a single s-sub shell containing a single s ... Orbital Diagram For Neon (Ne) | Neon Electron Configuration ... We shall also provide a broad representation of the element with its orbital diagram to make the things more appropriate for learning purposes. Neon is basically the name of a chemical element that is prominently part of chemistry. The element has the atomic number 10 and the representative symbol Ne. Orbital Diagram For Xenon - Wiring Diagrams Orbital Diagram For Xenon Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of xenon (atomic number: 54), an isotope of this. Sep 8, TL;DR Xenon hexafluoride has a fluxional structure in the gas phase, with . the best explanation is given by qualitative molecular orbital theory.
Orbital diagram for neon. PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4. Atom Diagrams: Electron Configurations of the Elements For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. Neon Bohr Model - How to draw Bohr diagram for Neon(Ne) atom Electron dot diagram of a Neon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Neon, we got to know, it has 8 valence electrons. So, just represent these 8 valence electrons around the Neon atom as a dot. Solved Fill in the orbital energy diagram for the neon ... Science. Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for the neon atom. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining.
Orbital Diagrams, Rules & Principles Quiz - Quizizz 30 seconds. Q. What is incorrect about this orbital diagram? answer choices. Both arrows in the 2p box should be pointing up. There is nothing incorrect with this diagram. In the 2p box there should only be 1 electron in the first 2p box and one in the 2nd 2p box. All the arrows should be pointing up. Tags: Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
What is neon orbital notation? - Quora Answered 5 years ago · Author has 109 answers and 561.3K answer views Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. Neon Orbital diagram, Electron configuration, and Valence ... The orbital diagram for Neon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Neon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the remaining six electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Neon atom is shown below- Properties and Uses of Neon Arrangements of electrons in the orbitals of an atom is ... The orbital diagrams for fluorine and neon are shown. The next two electrons continue to pair those electrons that are unpaired to fill up the 2p orbitals. With neon the second level is filled with electrons. Completed levels are a characteristic of all noble gases. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
Draw An Orbital Diagram For Boron. Draw dot diagrams for elements. .. Boron Through Neon - The 2p Orbitals Draw orbital diagrams and then the electron configurations for the following atoms. The number of electrons in an atom of an element is equal to the at number. the packing of the shell is: 1st (s2) 2nd (s2p6) 3rd (s2p6d10).
Orbital Diagram Practice | Chemistry Quiz - Quizizz Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
How to Write the Orbital Diagram for Neon (Ne) - YouTube To write the orbital diagram for the Neon atom (Ne) first we need to write the electron configuration for just Ne. To do that we need to find the number of ...
Orbital Diagram Of Neon - schemacheck.com An atomic orbital can hold a minimum of 6 electrons, each with opposite spins. An atomic orbital can hold a maximum of 6 electrons, each with the same spin. Draw the orbital diagram of neon, then circle the electron in this diagram that corresponds to the following set of quantum numbers n=2 l=1 ml=0 ms=1/2; Question: Draw the orbital diagram ...
Neon(Ne) electron configuration and orbital diagram Neon (Ne) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
What is the orbital diagram for neon? - Answers The orbital diagram for its shells is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. 2.0 engine and components diagram for a Plymouth neon? i need a timing belt alingment diagram of 1996 plymouth neon 2.0
Electron Configuration for Neon (Ne) - UMD How to Write the Electron Configuration for Neon. Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital.
Manganese(Mn) electron configuration and orbital diagram Manganese (Mn) excited state electron configuration and orbital diagram. When a manganese atom is excited, then the manganese atom absorbs energy. As a result, an electron in the 4s orbital jumps to the 4p x sub-orbital. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
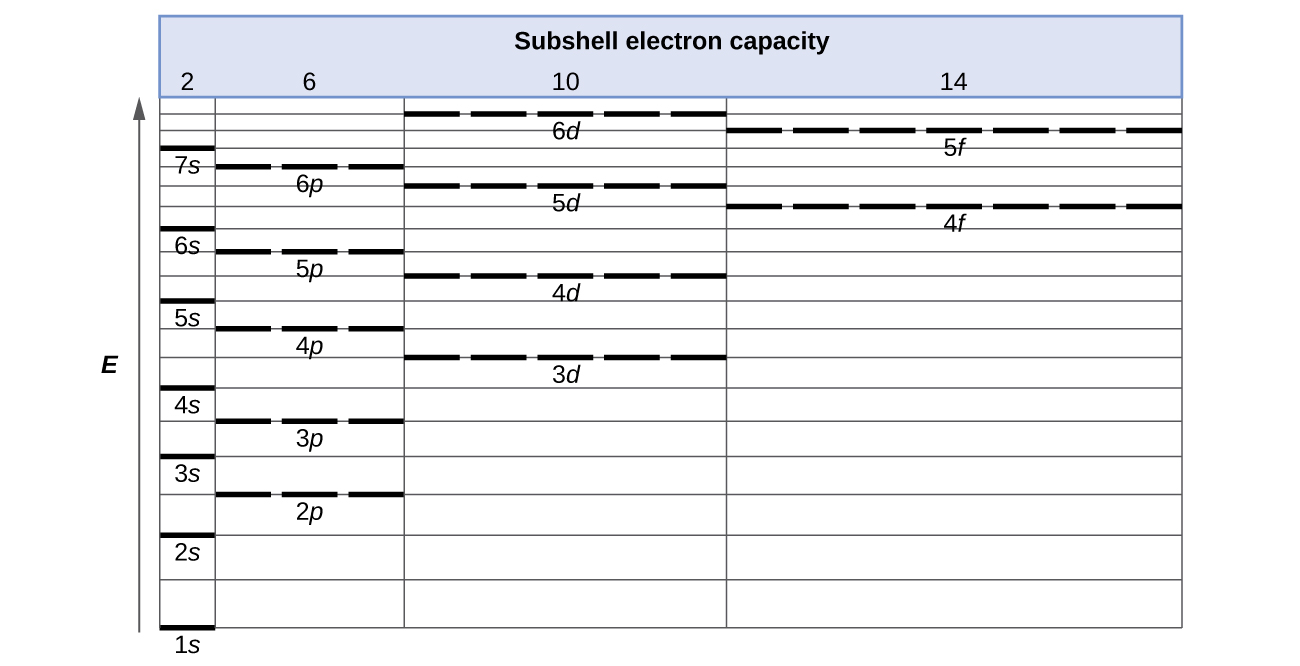
Orbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Dublin Schools - Lesson : Orbital diagrams and Electron ... Neon is the first element with a stable octet with complete p and s orbitals. The reason the octet is so stable is that the only way to add electrons is to add the next s orbital outward. There is no way to add electrons to the 2 shell of neon, period. Period 3 (Sodium to Argon)
Orbital Diagram For Xenon - Wiring Diagrams Orbital Diagram For Xenon Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of xenon (atomic number: 54), an isotope of this. Sep 8, TL;DR Xenon hexafluoride has a fluxional structure in the gas phase, with . the best explanation is given by qualitative molecular orbital theory.
Orbital Diagram For Neon (Ne) | Neon Electron Configuration ... We shall also provide a broad representation of the element with its orbital diagram to make the things more appropriate for learning purposes. Neon is basically the name of a chemical element that is prominently part of chemistry. The element has the atomic number 10 and the representative symbol Ne.
What is the electron configuration of the neon atom ... [2,8] or 1s^2 2s^2 2p^6 Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. The n=1 shell can only hold 2 electrons, so the remaining 8 electrons fill the n=2 shell. The more detailed version of the electronic configuration reflects the sub-shells in the n=1 and n=2 shells, where the n=1 shell just has a single s-sub shell containing a single s ...





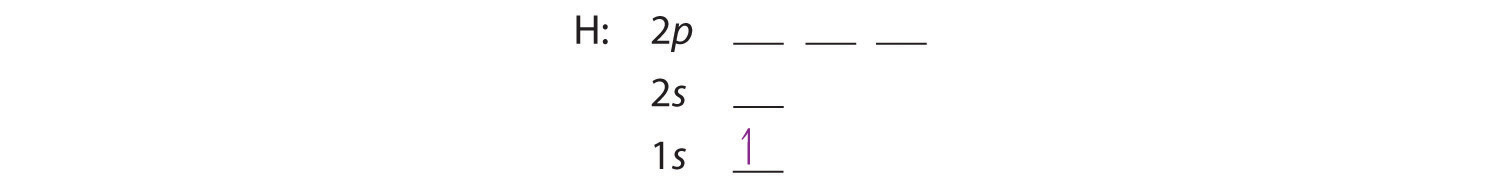

















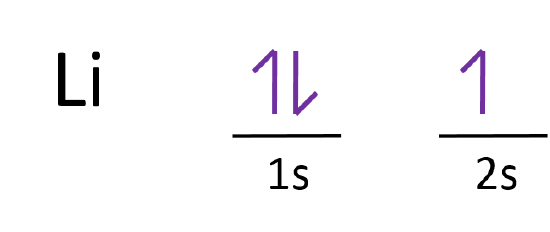



0 Response to "37 orbital diagram for neon"
Post a Comment