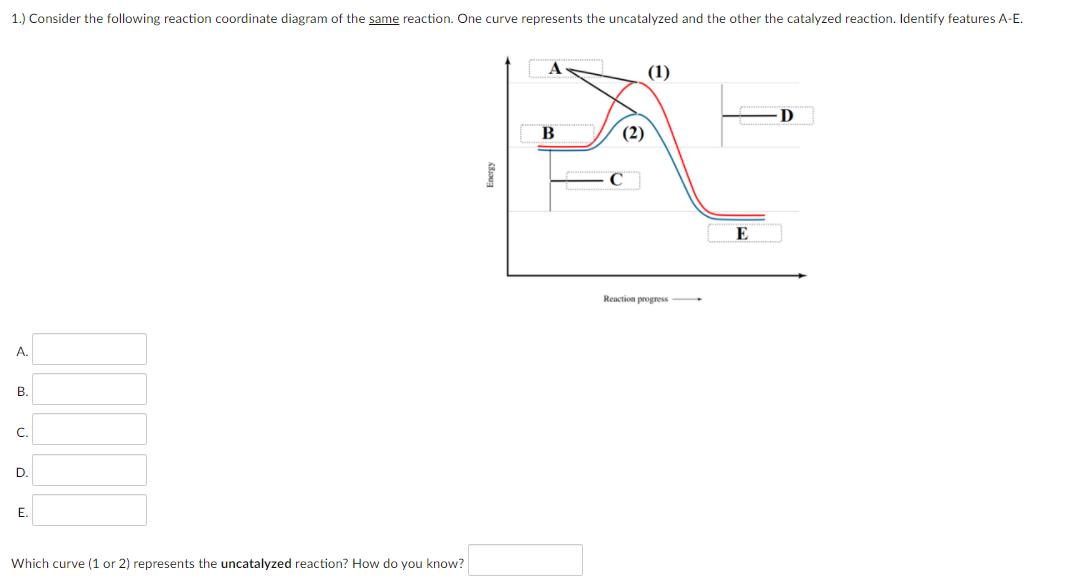
40 given the following reaction determine the correct reaction coordinate diagram
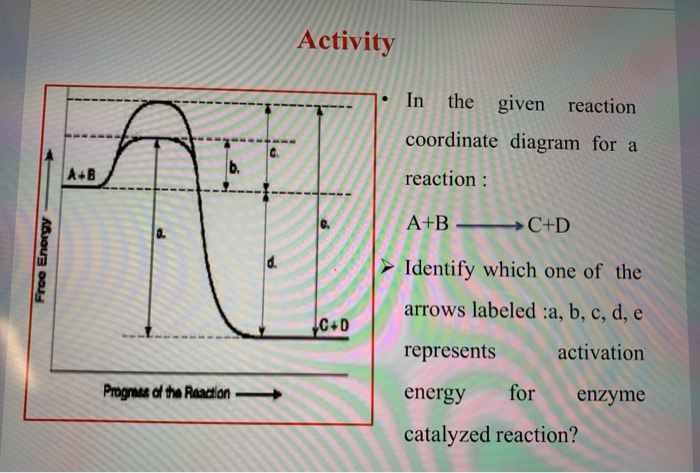
Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution A catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; they are, as we would expect, identical in that respect. The reaction coordinate diagram shows that the energy of activation for the reverse reaction is lowered by the catalyst as well. Enzymatic catalysis The ability of enzymes to catalyze reactions depends on their ability to interact directly and specifically with reactants The reactant of an enzyme-catalyzed reaction is termed the substrate .
Please do it on Priority. Transcribed Image Text: (a) Make Free Body Diagram (b) Find all the reactions labelled (c) Find the internal Shear and Moment Equation at Point C (d) Draw correct shear and bending Moment diagram. 25 Ib/ft 15 Ib/ft 50 lb-ft 2ft 40 lb Oft 4ft 6ft 3ft 2ft. Expert Solution.
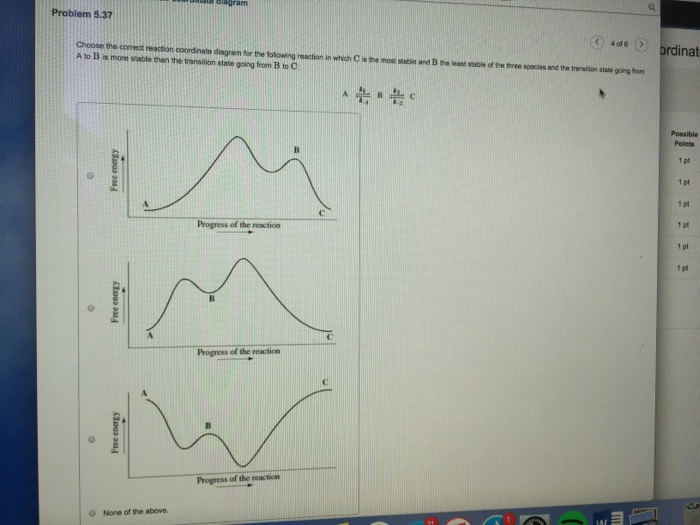
Given the following reaction determine the correct reaction coordinate diagram
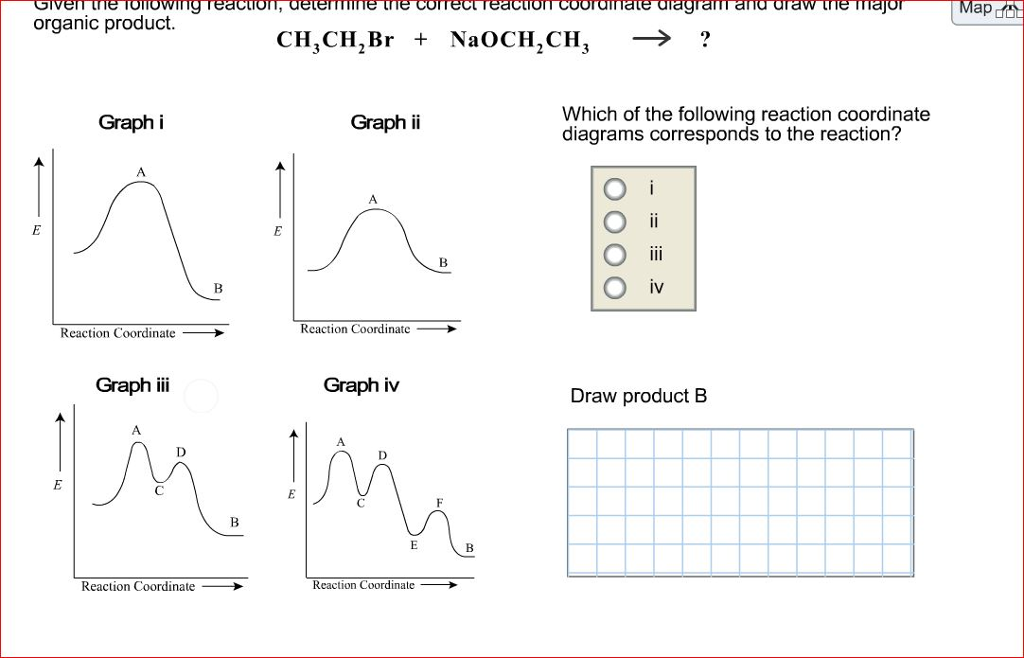
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed. Science. Chemistry. Chemistry questions and answers. Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product. CH_3 CH_2 Br + NaOCH_2 CH_3 rightarrow ? Which of the following reaction coordinate diagrams corresponds to the reaction? i ii iii iv Draw product B. Reaction coordinate diagrams clearly show that the energy of an enzyme bound to a transition state is higher than the energies of the E + S, E + P, and ES that occur along the same reaction coordinate. The energy of an enzyme bound to a transition state analog would lie _____ in the diagram. a. above the E + S but below the transition state
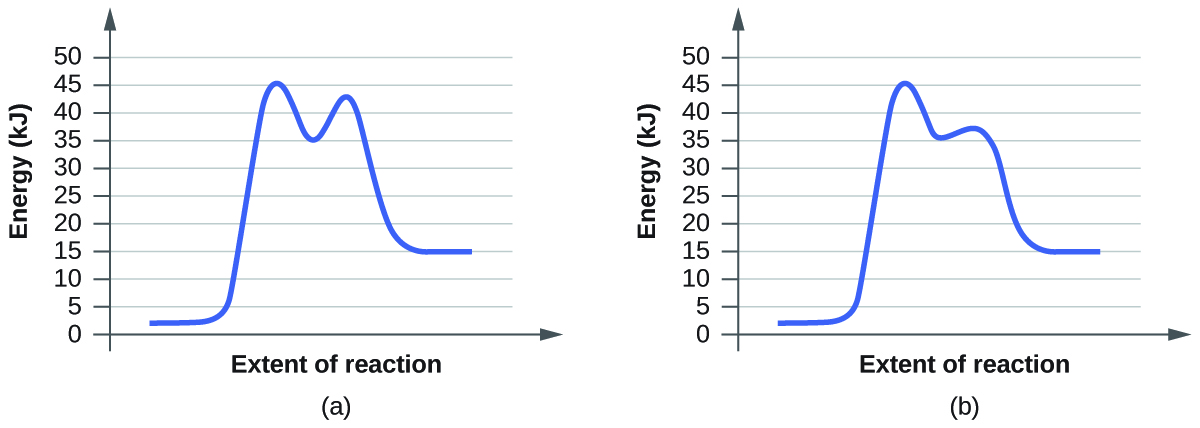
Given the following reaction determine the correct reaction coordinate diagram. Question: Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product. Which of the following reaction coordinate diagrams corresponds to the reaction? Graph i Graph ii IV Reaction Coordinat e ㅡㅡ Reaction Coordinate Graph i Graph iv Draw product B Reaction Coordinate Reaction Coordinate . Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. Compare the two reaction coordinate diagrams below and select the answer that correctly describes their relationship. In each case, the single intermediate is the ES complex. (a) describes a strict "lock and key" model, whereas (b) describes a transition-state complementarity model. The activation energy can be determined using the equation: ln (k 2 /k 1) = E a /R x (1/T 1 - 1/T 2) where. E a = the activation energy of the reaction in J/mol. R = the ideal gas constant = 8.3145 J/K·mol. T 1 and T 2 = absolute temperatures (in Kelvin) k 1 and k 2 = the reaction rate constants at T 1 and T 2.
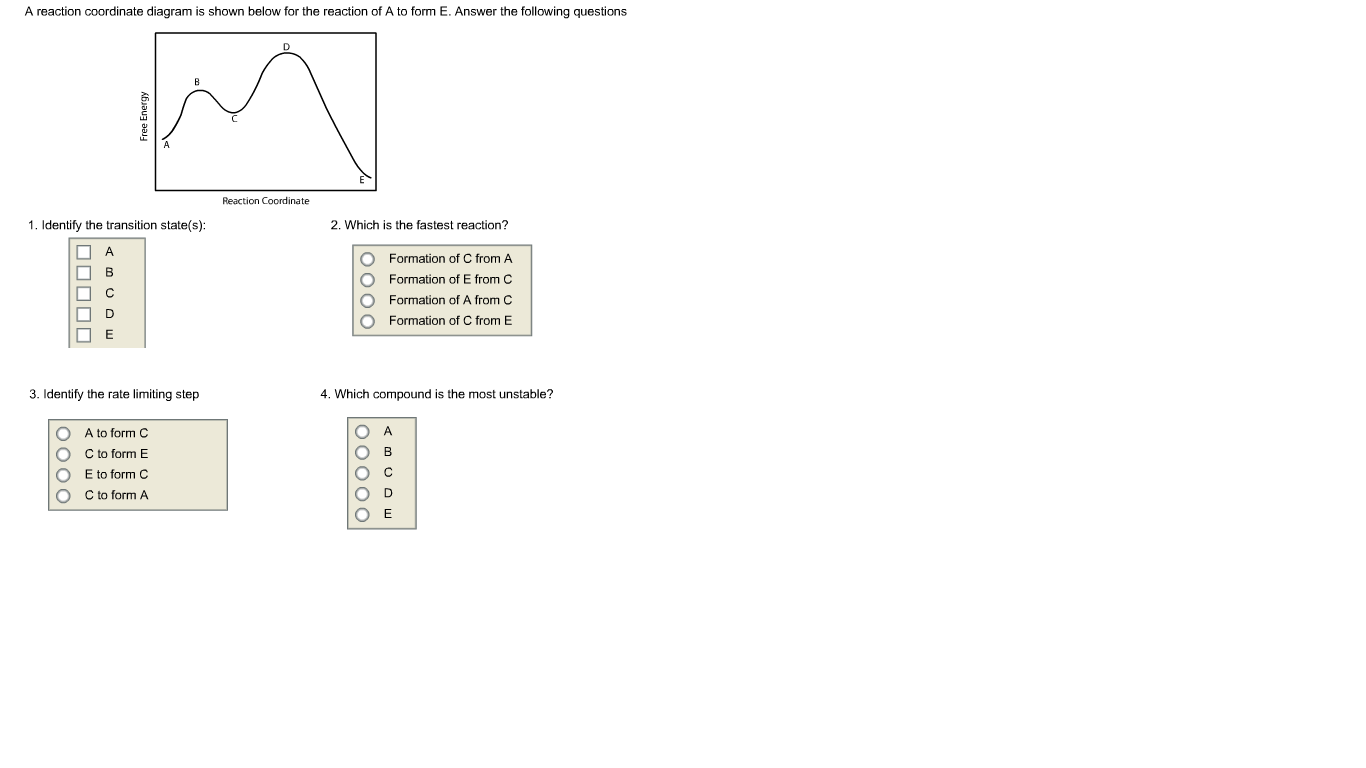
Consider the conversion diagram given below. At which point(s) in the diagram is a molar mass needed? ... Consider the following reaction coordinate diagram. ... Choose the correct equilibrium constant expression for the equilibrium 4 NO(g) + 6 H2O(g) 4 NH3(g) + 5 O2(g) ... Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b. Reaction Diagrams . Note that the x axis is officially titled "reaction coordinate." This is a somewhat complicated measure of how far the reaction has progressed, or how much of the reaction has happened. It is often more convenient to assume the reaction is "going" at a somewhat constant rate and think of the x axis simply as time. The potential energy diagram shown below represents the reaction a + b + 50 kJ c + Reaction Coordinate Given the potential energy diagram: Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain why.] Which lettered interval represents the potential energy of the actants of the
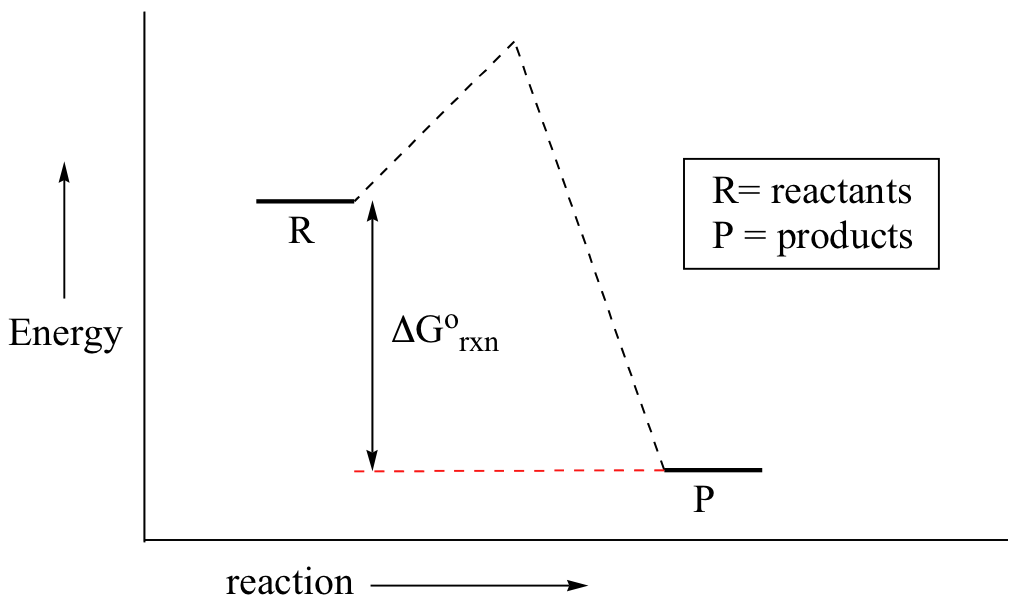
Exothermic reaction: heat is given off by the reaction (-ΔG˚)! Endothermic reaction: heat is consumed by the reaction (+ΔG˚)! Entropy (ΔS˚): a measure of the freedom of motion! - Reactions (and nature) always prefer more freedom of motion! Organic reactions are usually controlled by the enthalpy! 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Some of the amino acids in an enzyme's active site play a direct role in lowering the activation energy of the given reaction. ... Refer to the reaction coordinate diagram below. The change in the activation energy of the reaction because of the presence of an enzyme is illustrated by the energy of _____ ... Which of the following is correct ... Define spontaneity of a reaction in terms of ∆G. Determine if a process is spontaneous based on graphs or calculations. Identify which of the following reaction profile diagram is for an endergonic reaction.
This can be activation energy diagram for addition of HCl to 3,3-dimethyl-1-butene. Hard. Open in App Open_in_app. Solution. Verified by Toppr. Correct ...1 answer · Top answer: (a, b) B and C are places for reactive intermediates. D has lower potential enegy than A.It means energy released so exothermicHence options A,B are correct.Missing: determine | Must include: determine
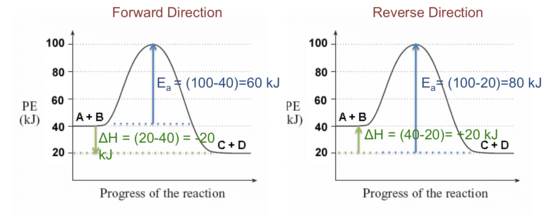
406 M-1 min-1 and the rate constant for the reverse reaction is 244 M-1 min-1. The activation energy for the forward reaction is 26.2 kJ mol-1 and that for the reverse reaction is 42.4 kJ mol-1. (a) (5 points) On the axes below, draw a reaction coordinate diagram for this reaction, showing the
See the answer. See the answer See the answer done loading. Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product.Which of the following reaction coordinate diagrams corresponds to the reaction?Draw product B. Expert Answer.
FREE Answer to Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product....1 answer · 1 vote: CH₂ CH BY & Na oct SN CH2 CH20-4 CH 3 product (B) This Reaction can be proceed via SM² mechanism so sna is one step mechanism and No Ontermediate is ...
1. Non-elementary steps, or complex reactions, are sets of elementary reactions. The addition of elementary steps produces complex, non-elementary reactions. 2. The correct statements are "a" and "e". By definition of elementary reactions they have 0 intermediates because they cannot be broken down. Again by definition of an elementary reaction ...
Consider the following diagrams which show the progress for the reaction A (blue) ⇌ B (red). The equilibrium constant (K) for this reaction is 0.8. At which point does the reaction reach equilibrium? The equilibrium constant is the products divided by the reactants. A K value of 0.8 is consistent with image choice C where the ratio of product ...
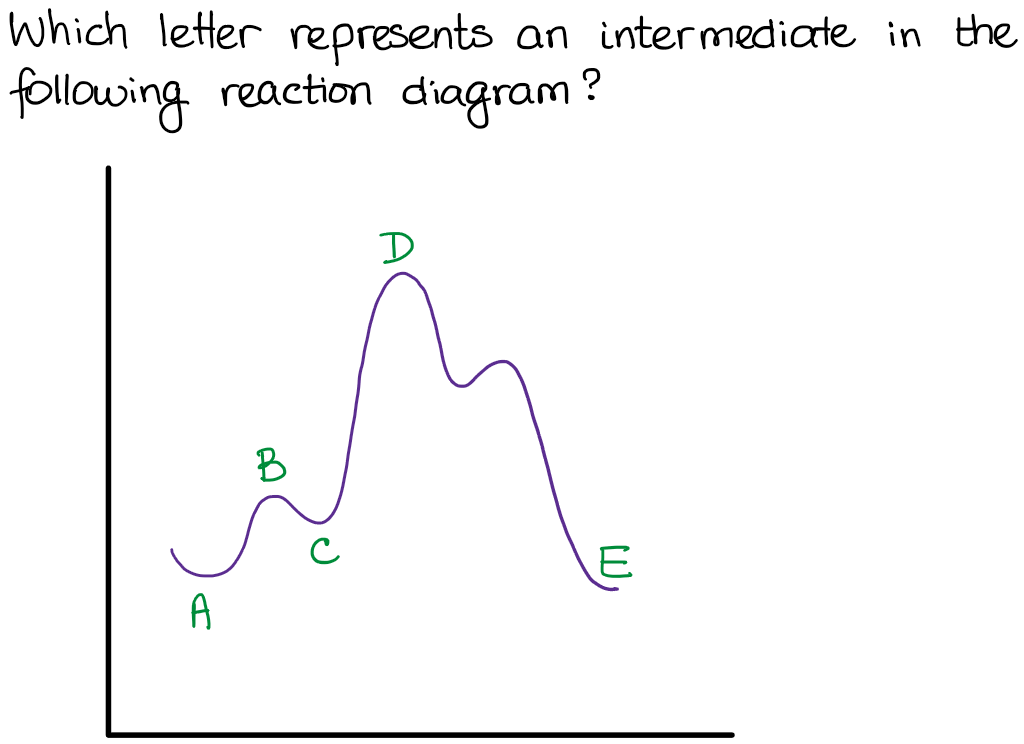
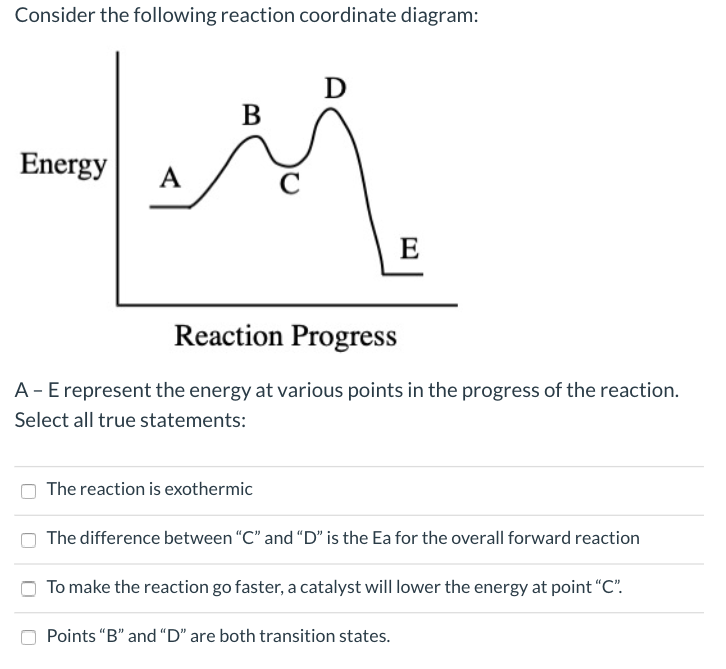
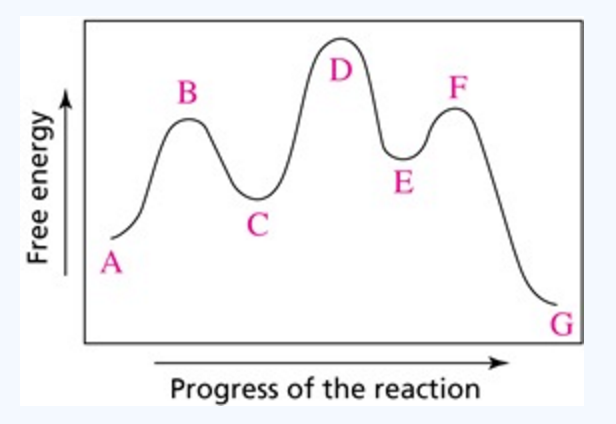
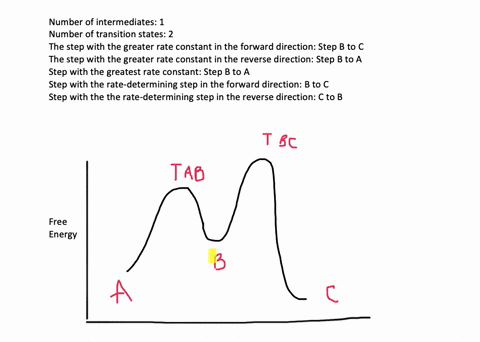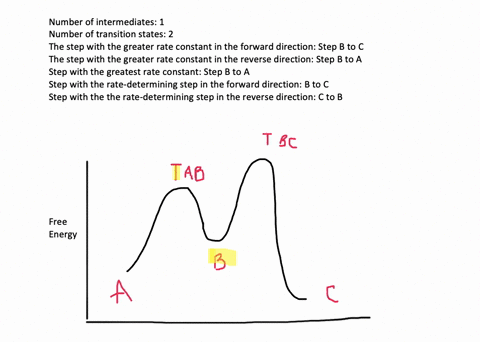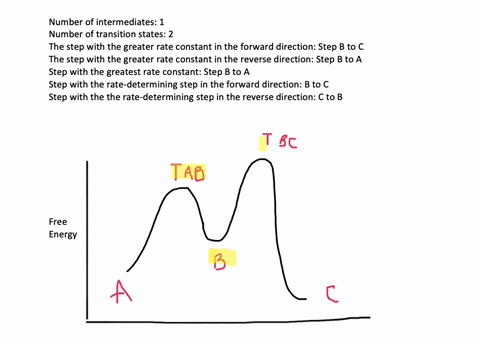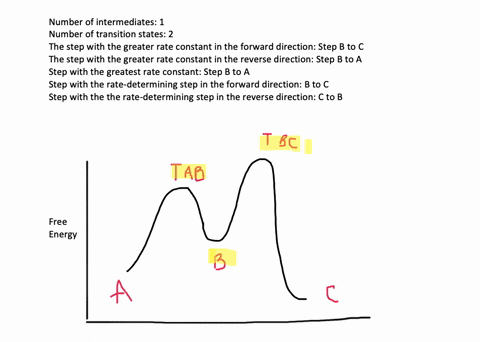
According to Wikipedia: Given a reaction coordinate (energy diagram), the rate determining step can be determined by taking the largest energy difference between any starting material or intermediate on the diagram and any transition state that comes after it. That transition state will then be the rate-determining step of a given reaction.
Solution Verified by Toppr Correct option is D Solution:- (D) The given reaction is followed by SN1 mechanism. The reaction proceeds in two steps - a slow step (high activation energy) followed by a fast step (low activation energy). Thus the (D)is the correct coordinate diagram for the given solvolysis reaction. Was this answer helpful? 0 0
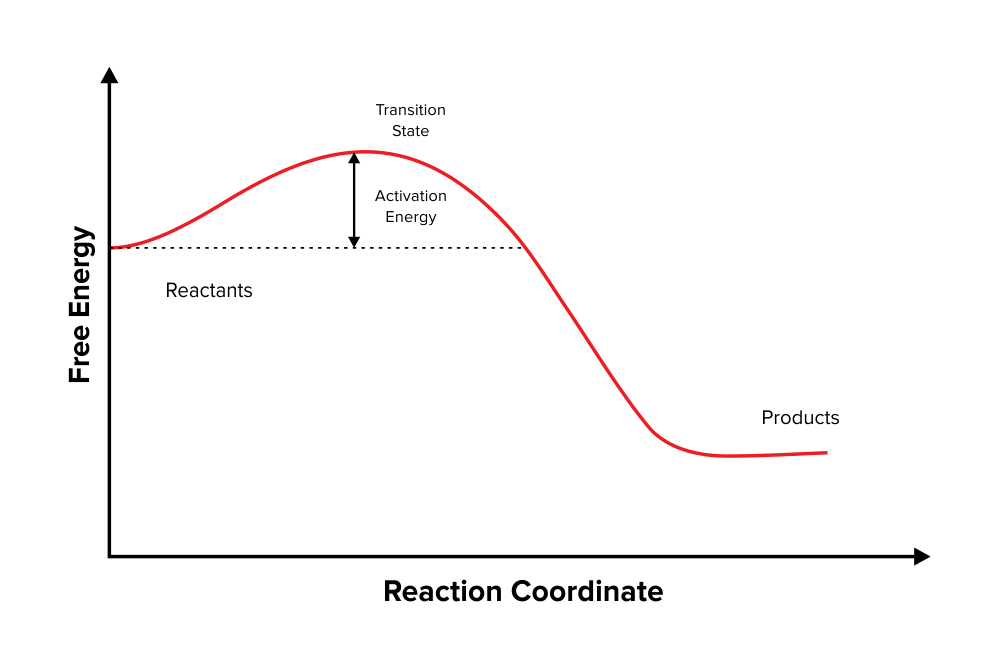
Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 .
Question: Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product. CH, CH,Br + NaOCH,CH, → ? Graphi Graph ii Which of the following reaction coordinate diagrams corresponds to the reaction? Oooo Reaction Coordinate Reaction Coordinate Graph iii Graph iv Draw product B Reaction Coordinate.
Reaction coordinate diagrams clearly show that the energy of an enzyme bound to a transition state is higher than the energies of the E + S, E + P, and ES that occur along the same reaction coordinate. The energy of an enzyme bound to a transition state analog would lie _____ in the diagram. a. above the E + S but below the transition state
Science. Chemistry. Chemistry questions and answers. Given the following reaction, determine the correct reaction coordinate diagram and draw the major organic product. CH_3 CH_2 Br + NaOCH_2 CH_3 rightarrow ? Which of the following reaction coordinate diagrams corresponds to the reaction? i ii iii iv Draw product B.
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed.



















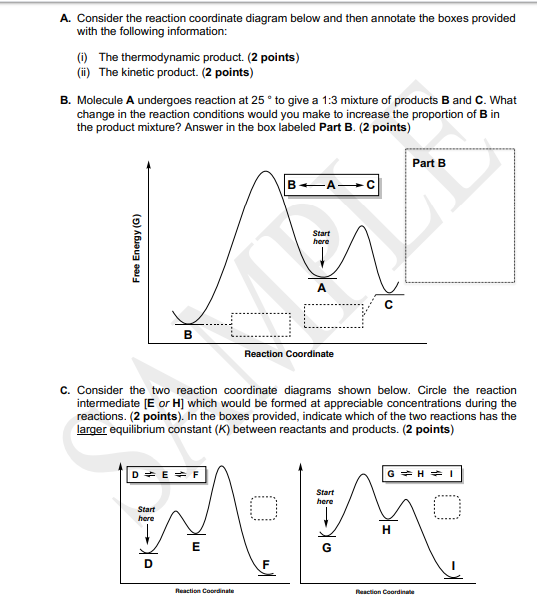
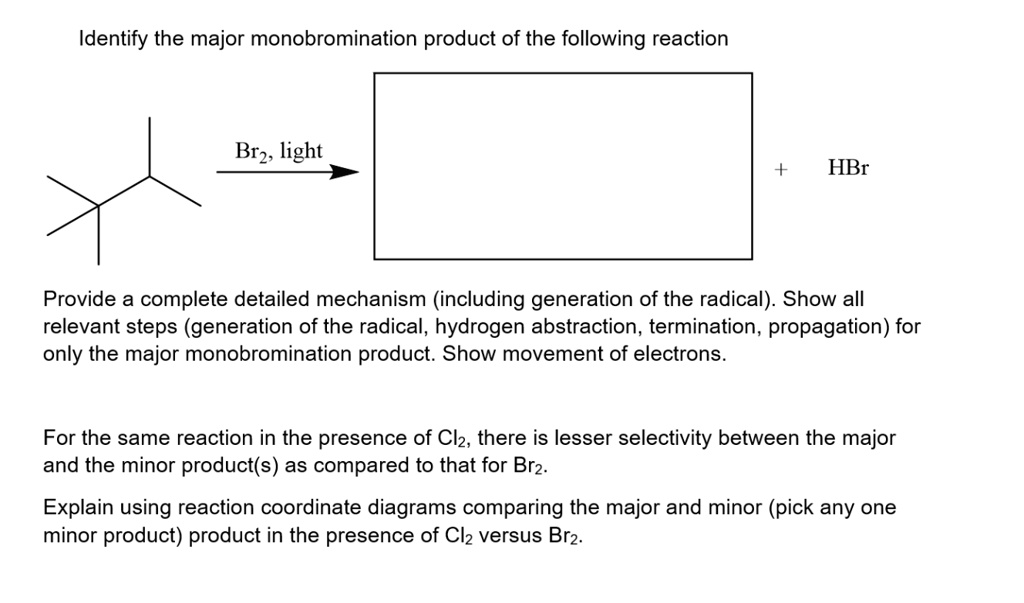
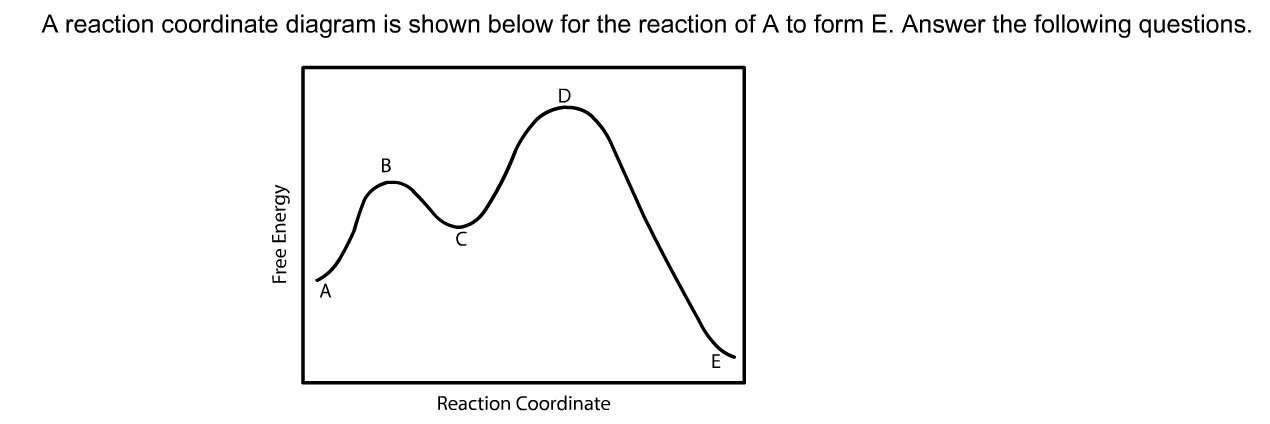
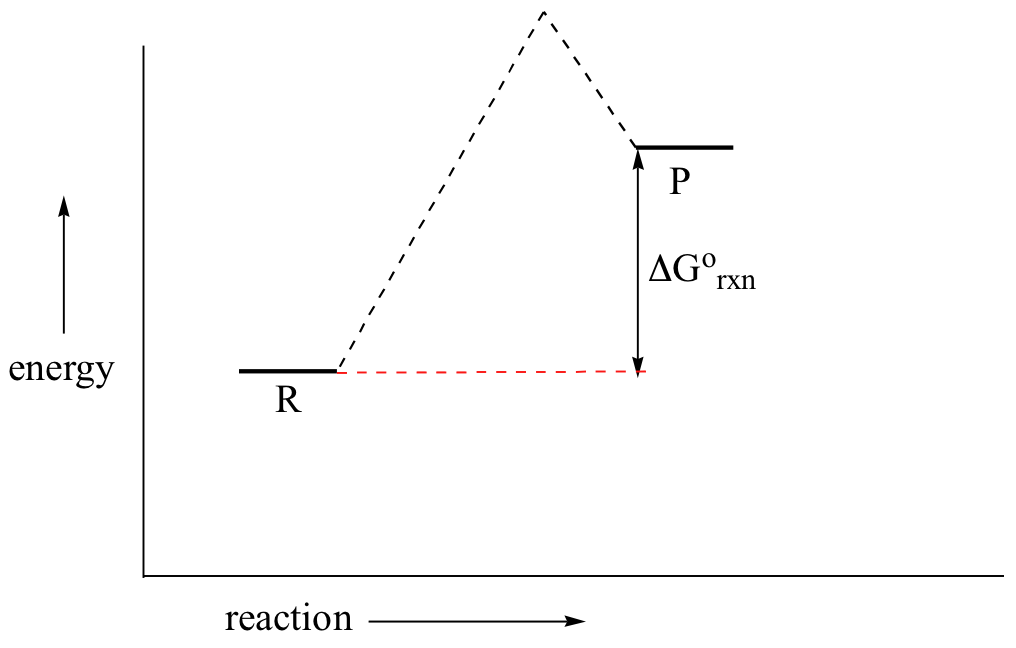


0 Response to "40 given the following reaction determine the correct reaction coordinate diagram"
Post a Comment