40 molecular orbital diagram problems
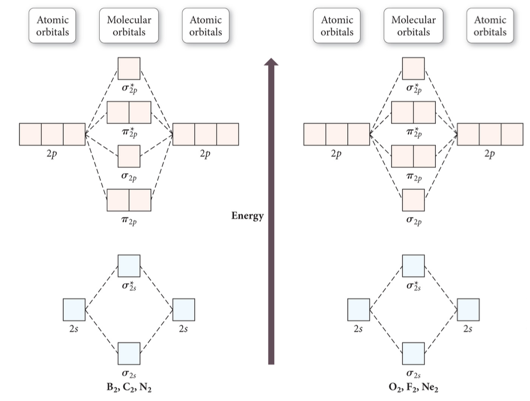
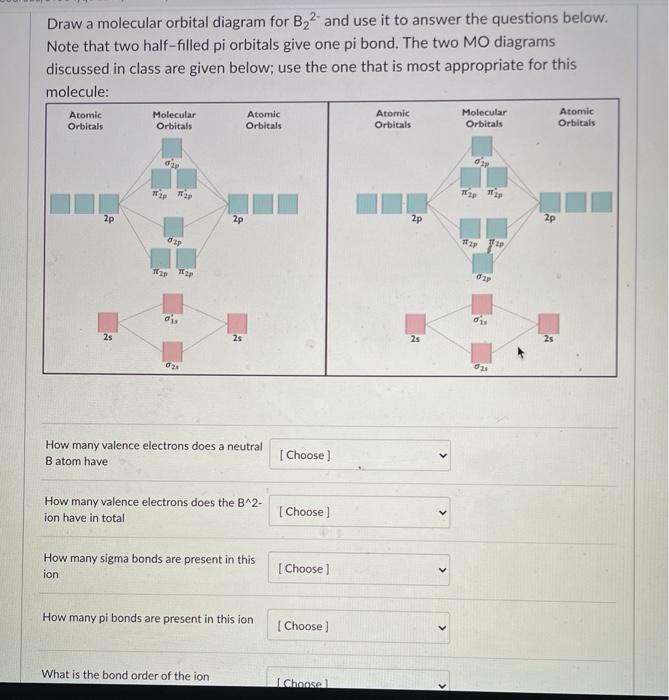
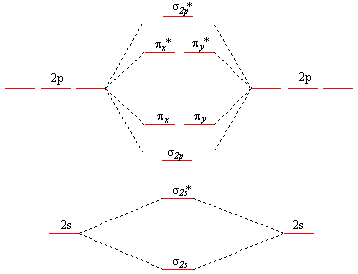
Molecular Orbitals: Problems and Solutions | SparkNotes By constructing a molecular orbital picture for each of the following molecules, determine whether it is paramagnetic or diamagnetic. a. B 2 b. C 2 c. O 2 d. NO e. CO a. B 2 is paramagnetic because it has two unpaired electrons, one in each of its p orbitals. b. C 2 is diamagnetic because all of its electrons are paired. Molecular Orbital Theory Practice Problems - XpCourse Live www1.lasalle.edu Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H More ›
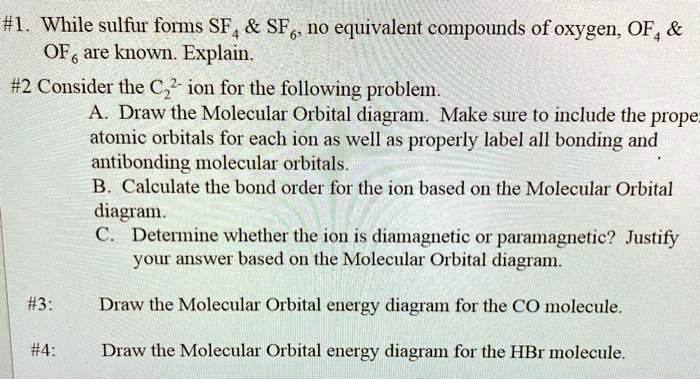
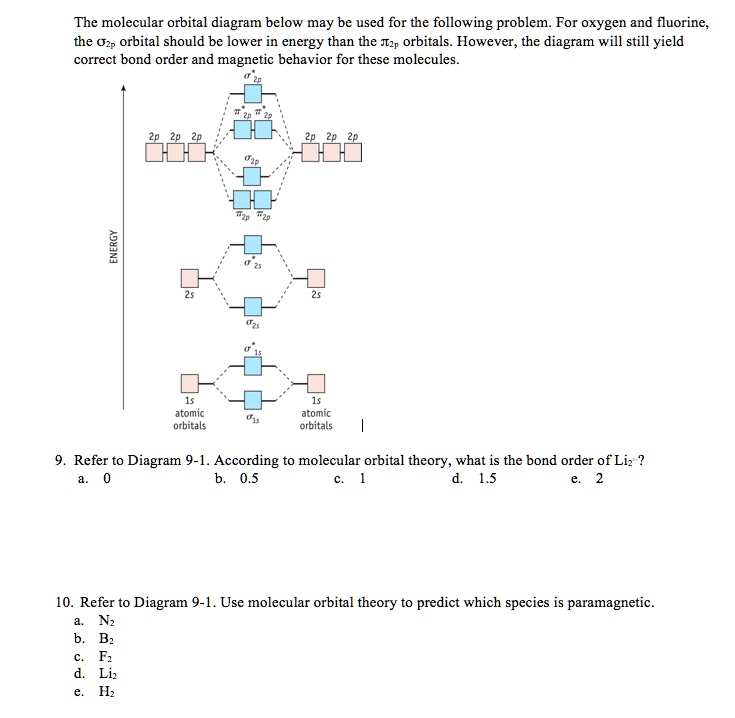
Chapter 9, Molecular Orbitals in Chemical Bonding Video ... Problem 1 Describe the main differences between the valence bond theory and the molecular orbital theory. Natalie J. ... Assume the molecular orbital diagram for a homonuclear diatomic molecule (Figure $9-5($ a $)$ ) applies to $\mathrm{NO}^{+}$. What is the highest-energy molecular orbital occupied by electrons?

Molecular orbital diagram problems
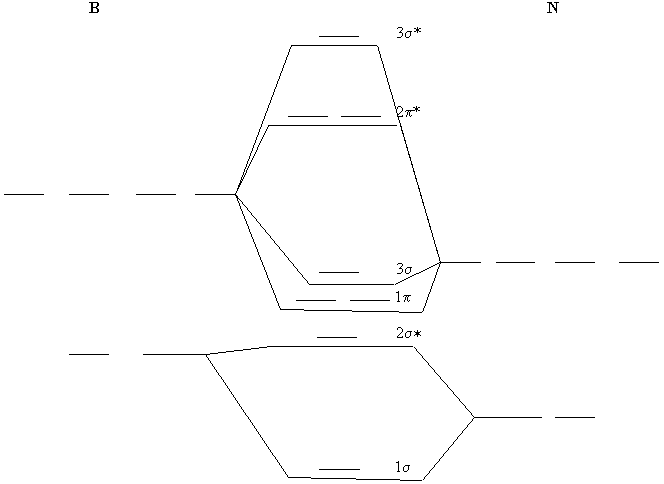
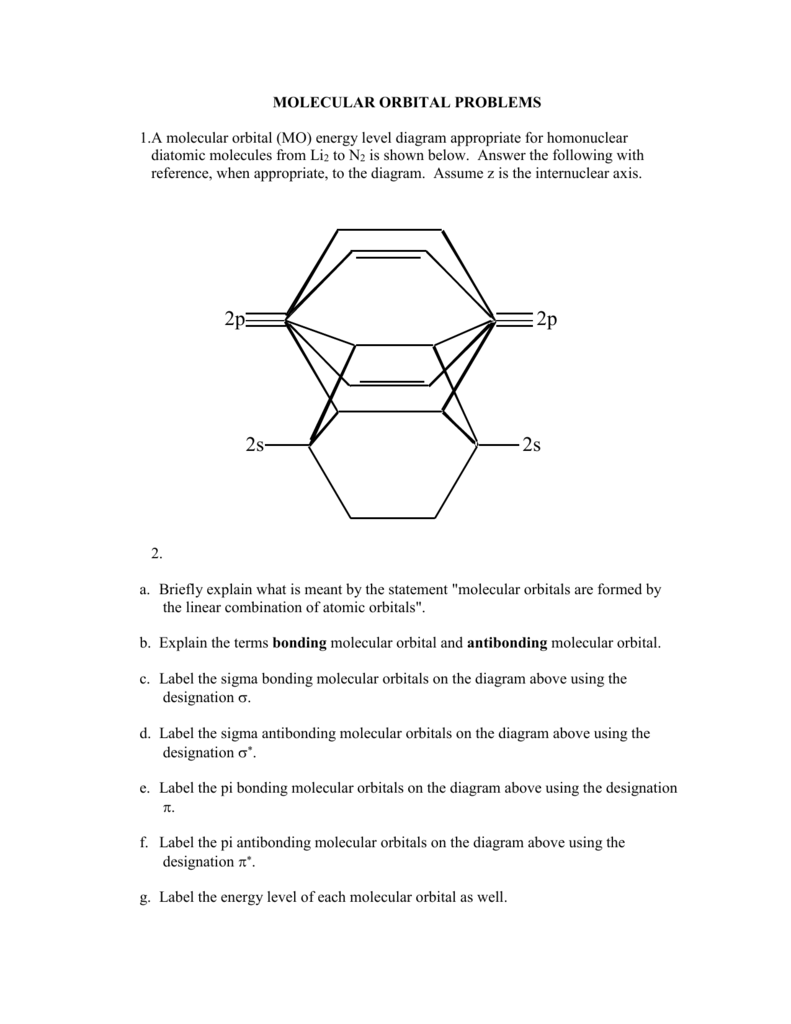
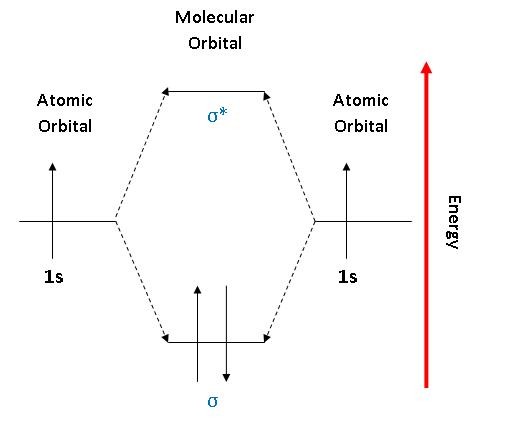
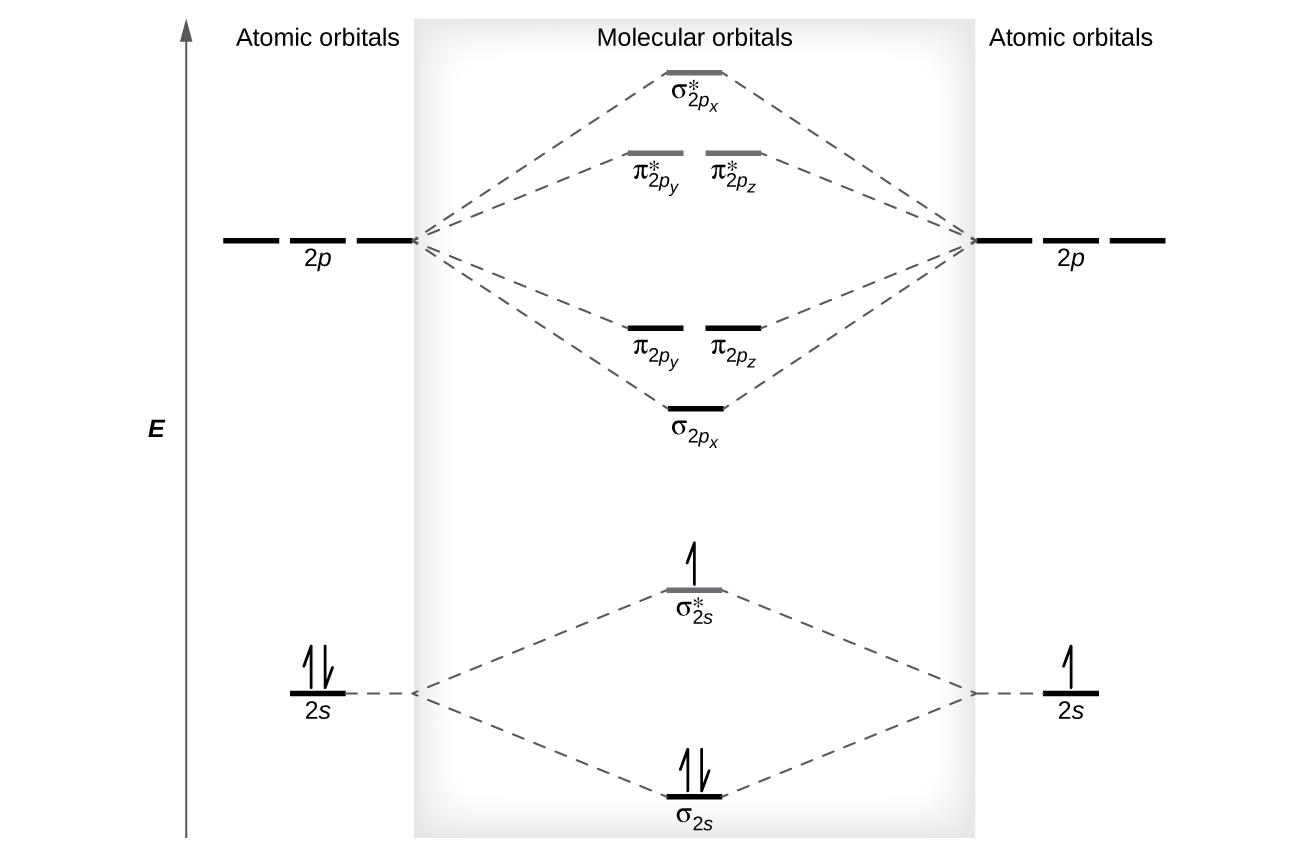
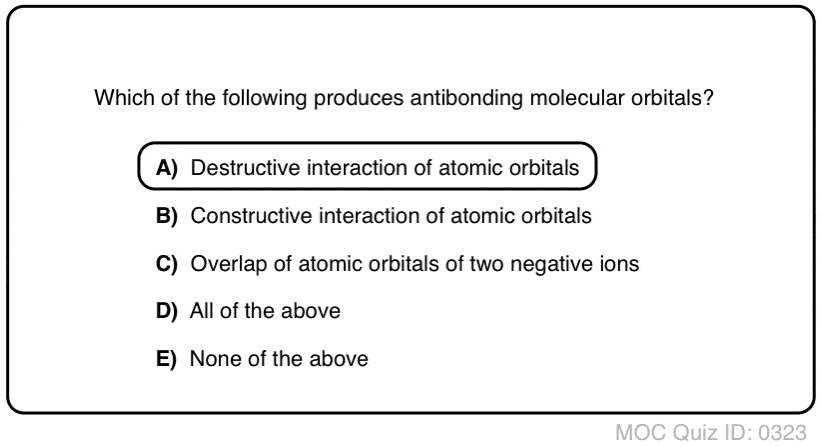
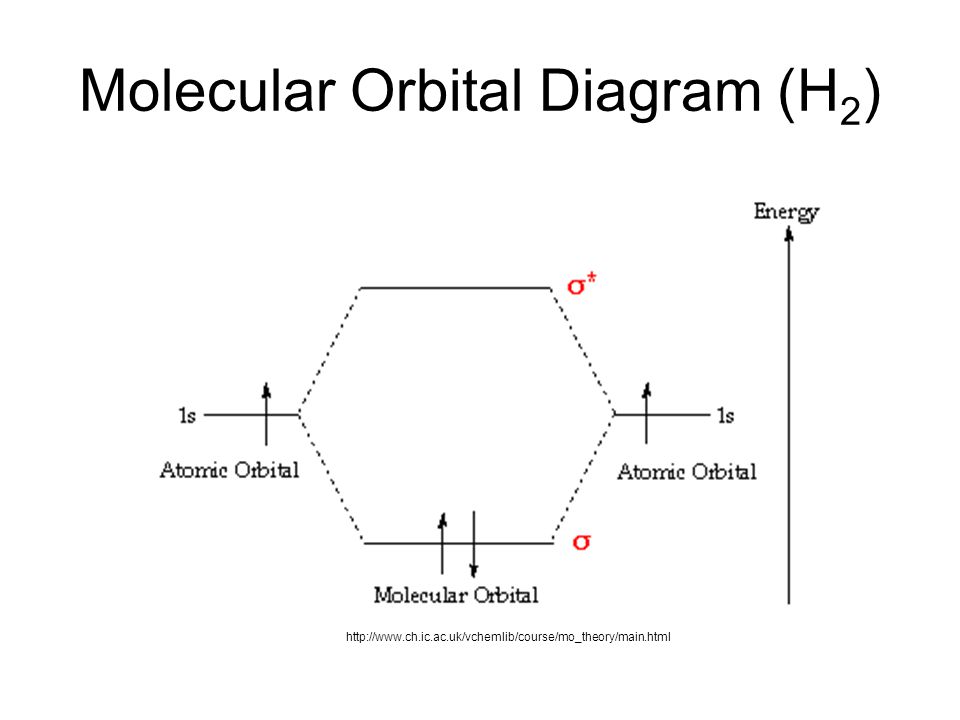
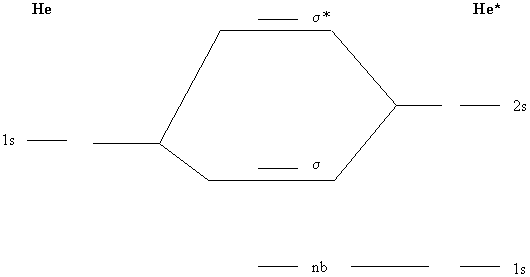
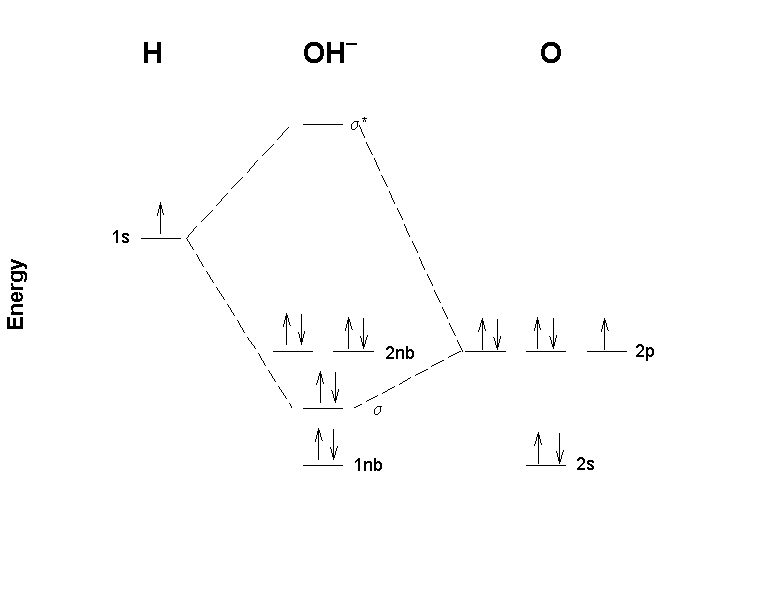
PDF 1 Lecture 2 Simple Molecular Orbitals - Sigma and Pi Bonds ... Pi star (π*): antibonding molecular orbital - Normally this orbital is empty, but if it should be occupied, the wave nature of electron density is out of phase (destructive interference) and canceling in nature. This produces repulsion between the two interacting atoms, when electrons are present. Atoms gain a lot by forming molecular orbitals. 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Molecular orbital diagram problems. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. PDF 1 Lecture 2 Simple Molecular Orbitals - Sigma and Pi Bonds ... Pi star (π*): antibonding molecular orbital - Normally this orbital is empty, but if it should be occupied, the wave nature of electron density is out of phase (destructive interference) and canceling in nature. This produces repulsion between the two interacting atoms, when electrons are present. Atoms gain a lot by forming molecular orbitals.












0 Response to "40 molecular orbital diagram problems"
Post a Comment